Table of Contents
Effects of Cocaine on the Brain
Introduction
History
Cocaine (aka Benzoylmethyl ecgonine) is a stimulant drug that produces its effects by activating the brain’s natural dopaminergic reward pathway. It is a basic alkaloid derived from the leaves of the erythroxylon coca plant in South America, which humans have been consuming since approximately 3000 BC. In 1860 cocaine was isolated for the first time and became common in many practices such as medicine [1]. It’s various uses included but are not limited to – treating diseases, acting as a natural painkiller and anesthetic, and was even an ingredient in Coca Cola soda. However, due to its highly addictive nature, cocaine was made illegal in the mid 1900s and has since been classified as a Schedule 1 drug with high potential for addiction [1].
Cocaine is produced and abused in two forms. The first is a water soluble hydrochloride salt. This form is abused intranasally or intravenously. The second form of cocaine is a water insoluble base, in which users smoke the drug. This form is more commonly referred to as “”crack cocaine” [2].
Molecular Properties
<style justify>
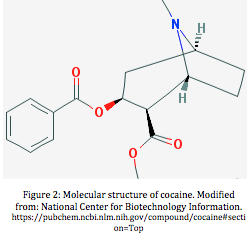
At the molecular level, cocaine consists of 3 functional groups, a lipophilic, hydrophilic and aliphatic group. Due to the presence of a hydrophilic functional group, cocaine is able to enter the blood stream and travel to the brain in the aqueous plasma. Cocaine’s ability to solubilize is also mediated by the amino and carbonyl functional groups, which allow it to hydrogen bond with water, thus contributing to its hydrophilicity. In addition, the lipophilic functional group of the molecule allows cocaine to cross the hydrophobic blood brain barrier where it mediates its effects on the brain. These functional characteristics are thus essential for cocaine’s transmission throughout the body and to its target organ [3].
The functional characteristics of cocaine allow it to be rapidly absorbed by the body. Inhalation (i.e. smoking) and injection provide the fastest routes of absorption, requiring only seconds to produce an effect; whereas intranasal administration requires approximately 5 minutes to produce an effect [4]. The body processes cocaine through the liver where it is demethylated into various metabolic variants. The two most common urinary metabolites of cocaine are Benzoylecgonine and ecgonine methyl ester, which are both inactive forms. These metabolites can be detected in urine 3-5 hours after drug use and consequently are used as markers of cocaine use during drug tests [5]. Thus cocaine is rapidly absorbed, metabolized and excreted.
</style>
Acute vs Chronic Effects
<style justify>
Cocaine acts on the brain’s limbic system by producing psychoactive and addictive effects. One initial short-term effect is the build-up of the neurochemical, dopamine in chemical synapses of the brain. This effect is what drives cocaine users to take the drug repeatedly due to the increasing levels of euphoria [6]. A high volume of research today is focused on understanding how cocaine’s many long term effects produce addictions, cravings and risk of relapse [6].
The effects of cocaine appear shortly after taking the first single dose and can disappear within a few minutes to an hour. The reality of cocaine hits after the high. Cocaine has powerful negative effects on the heart, brain and emotions. Many users become addicted quickly, with long term and life threatening consequences. Cocaine produces it's powerful “high” by acting on the brain but also affects the whole body as it travels through the blood. This drug is responsible for more U.S. emergency room visits than any other illegal drug [7]. Cocaine predominately harms the brain, lungs, neural vessels and heart. Cocaine acts in the deep areas of the brain. These are the areas that give an individual the feeling of “reward” from highly pleasurable behaviors. Stimulating these areas in the brain causes users to feel good and it can create a powerful craving to use more cocaine. Regular use can lead to tolerance. This means that increasingly higher doses are needed to attain the same effects, this often results in addiction [7].
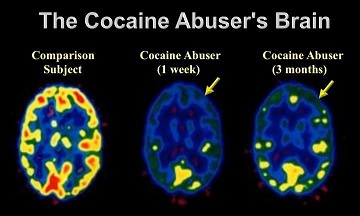
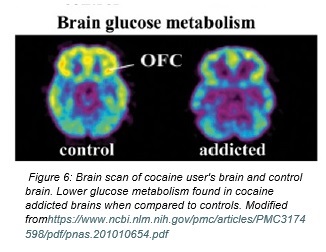
Figure 3 Low frontal metabolism may contribute to the loss of control seen in addiction
Brain: Cocaine effects the brain by causing blood vessels in the brain to constrict, which can therefore lead to a stroke. This is a common consequence of chronic cocaine use, even in young adults without other risk factors for strokes. Cocaine can also cause seizures and can lead to violent behaviour.
Lungs: The methods that are used to take this drug can cause damage to the respiratory system. Snorting cocaine damages the nose and sinuses. Smoking crack cocaine irritates the lungs and for some people it can lead to permanent lung damage.
Kidneys: Cocaine use can cause severely overwhelming kidney failure through a process called rhabdomyolysis . Users with high blood pressure that use cocaine regularly can accelerate the long term kidney damage caused by high blood pressure.
Sexual function: Chronic cocaine use can impair sexual function in men and women. In men, cocaine can cause delayed or impaired ejaculation.
Gastrointestinal tract: Cocaine can cause constriction in blood vessels supplying the gut. Therefore resulting in oxygen starvation which can cause ulcers or perforation of the stomach or intestines.
</style>
Mechanism of Action
Overview of the Limbic System
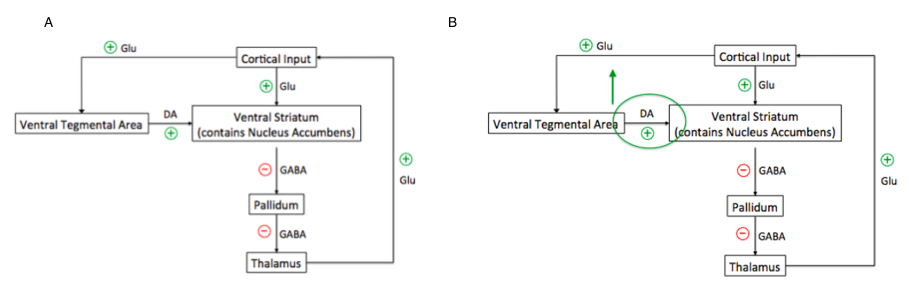
<style justify> Cocaine acts by affecting the limbic system in the brain, as shown below in Figure 4. The limbic system consists of several regions in the brain associated with emotions, such as fear and anger, pleasure-seeking behaviour (which is related to survival), such as eating and sex, and motivation [8]. Specifically, the subcortical structures and areas of the forebrain that are integrated into limbic circuitry include: the amygdala, hippocampus, basal ganglia, hypothalamus, cingulate gyrus, thalamus, and the pallidum. These areas are involved in peripheral nervous system and endocrine system activation. Normally, since the amygdala and hippocampus are involved with memory, they recognize which events or behaviours activate the reward circuitry in the limbic system, thus determining which events are emotionally significant [9]. The two components of the basal ganglia that are most affected by cocaine administration are the ventral tegmental area (VTA) and the ventral striatum, which contains the nucleus accumbens. During normal rewarding behaviours, the cortex stimulates these regions, activating the reward pathway. This is primarily done by stimulatory glutamatergic input sent to both the VTA and the ventral striatum. The VTA further stimulates the nucleus accumbens region of the ventral striatum through dopaminergic projection neurons. Specifically, these neurons in the nucleus accumbens region are GABAergic inhibitory neurons, called medium spiny neurons (MSNs). When stimulated, these inhibit the neurons of the Pallidum, which are tonically inhibiting the thalamus. By doing this, the inhibition onto the thalamus by the pallidum is stopped, allowing the thalamus neurons to be stimulated. This release of inhibition on the thalamus is called disinhibition. Stimulation of thalamic glutamatergic neurons positively feeds back into regions of the cortex, such as the motor cortex, amygdala and hippocampus With enough stimulation of this pathway, the behaviour or event that causes stimulation of the reward pathway is associated with feelings of pleasure, and the brain increases the frequency of the behaviour [8]. In the case of cocaine administration, cocaine increases the dopaminergic stimulation onto the nucleus accumbens, leading to disinhibition of the thalamus, and stimulation of the reward pathway. With enough stimulation, cocaine administration is paired with euphoric feelings, so the brain increases the frequency of behaviour, which can eventually lead to addiction [9].
</style>
Figure 4A: Normal activity of the limbic system contrasted with B: enhanced dopaminergic signalling due to cocaine administration from the ventral tegmental area to the nucleus accumbens regions of the basal ganglia
Cocaine's Effect on Dopaminergic Neurons of The Basal Ganglia
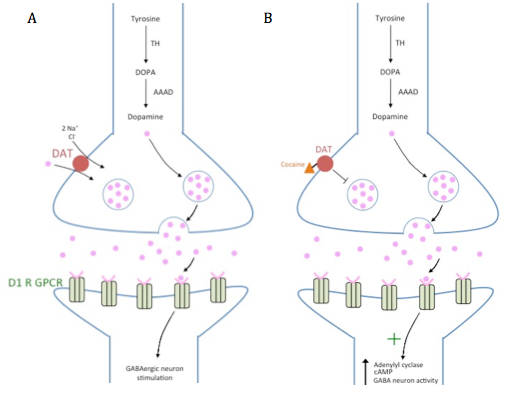
Specifically, cocaine acts as a monamine uptake inhibitor on the dopaminergic projection neurons from the VTA, which synapse with GABAergic MSNs in the nucleus accumbens, as shown in Figure 5. the dopamine receptors present on the post-synaptic terminals of the MSNs are D1 (dopamine 1) G-protein coupled receptors (GPCRs). D1 GPCRs are stimulatory since they are coupled to the G-protein Gs (stimulatory) which activates the adenylyl cyclase pathway, increases cAMP concentration, and thereby increases GABAergic neuron stimulation [8]. To help stop the signal, a dopamine transporter called DAT uses the energy from sodium and chloride ions travelling down their concentration gradient, to co-transport dopamine back into the neuron. Cocaine competitively inhibits DAT, preventing dopamine reuptake, and keeping it in the synaptic cleft for a longer amount of time. This allows prolonged stimulation of GABAergic neurons in the striatum. Increased GABAergic signalling in the striatum eventually leads to disinhibition of the thalamus (i.e. the inhibition onto the thalamus is removed). The thalamus can then stimulate the amygdala, which registers cocaine administration as an emotionally significant effect. It also stimulates areas of the cortex, such as the motor cortex, which increases the action of taking cocaine, as it is seen as a pleasure-seeking behaviour. Ultimately, this increases the desire to take more cocaine, which can potentially lead to cocaine addiction [9].
Figure 5: A dopaminergic synapse from the ventral tegmental area (VTA) onto the GABAergic medium spiny neurons of the nucleus accumbens. A: Normal dopamine reuptake by dopamine transporter (DAT) from the synaptic cleft into the pre-synaptic terminal, contrasted with B: the presence of cocaine which blocks DAT, preventing this dopamine reuptake mechanism.
Changes in Brain Morphology after Cocaine usage
Extensive research has been carried out in studying the effects of cocaine on the morphology of the brain, yet many discrepancies exist in the clinical literature as to what specific parts of the brain are influenced by cocaine usage. A multitude of studies have found differences in in the amount of grey and white matter when comparing the cocaine-dependent brains and brain tissue of control groups, yet these inconsistencies make it difficult to determine exactly what regions are affected [10]; nonetheless, the nucleus accumbens, the cerebellum, and the orbitofrontal cortex are three regions that have become apparent as the areas most often affected by cocaine administration.
The nucleus accumbens plays a central role in the reward circuitry of the brain [11], and has been determined to be the “most important site of cocaine high” [12]. A 2001 study by the University of Michigan and the University of Lethbridge investigated the influence of cocaine usage on the nucleus accumbens. They executed this research by allowing the rats in the experimental group to self-administer cocaine for 1 hour a day for one month, and left the control group undisturbed for the same period of time. From this study, the researchers determined that rats who self-administered cocaine experienced an increase in the dendritic branching and an increased dendritic density in the nucleus accumbens [12]. Although the exact mechanism of how cocaine triggers the nerve cells in the nucleus accumbens to branch off and extend is still unknown, it has been hypothesized that this change will pick up more signals from other regions such as the hippocampus, the amygdala, and the frontal cortex [12]. This allows these regions to have greater control over the functions of the nucleus accumbens, causing long-standing changes in behaviour such as intense drug cravings when drug-associated memories are stimulated [11].
The cerebellum, a region of the brain involved in motor control and movement coordination, has also been found to be impacted by chronic exposure to cocaine [13][14]. Specifically, multiple studies have reported cocaine users to experience an increase in cerebellar activity. A 2014 study on the effect of cocaine on task execution in rhesus monkeys compared the working memory performance between a group that had previously self-administered cocaine for 12 months, and a control group that self-administered water for that length of time. Both groups were drug-free for 20 months prior to the start of the study. The researchers found no differences in the execution of the tasks, but detect an increase in cerebellar metabolic activity in the cocaine group whilst executing the aforementioned tasks. This finding matches the results of previous literature [13], and has been previously suggested to be a means of compensating for hypoactivity in cortical areas of the brain [10].
Finally, the orbitofrontal cortex (OFC) is a critical structure in the process of decision making and emotional regulation [15]. A number of studies have found a decrease in grey matter in this brain region with the chronic usage of cocaine, which has been used hypothesized to cause behavioural changes in users, such as engaging in risky behaviour [16]. A 2014 study done by Federica Lucantonio and collegues compared the synaptic activity in the orbitofrontal cortex between rats fed cocaine for 14 days, and a control group that was trained to self-administer oral sucrose for the same amount of time [17]. The rats were then trained with a Pavlovian-style task, where they were taught to respond to several cues by means of reward. This training required the rats to use insight to determine the likely outcomes of an event in order guide their response. The control group was found to experience change in neural activity in the OFC between task training- specifically, an increased conditioned response which led to the appropriate behaviour. This confirmed that the neural activity in the orbitofrontal cortex induced the resulting behaviour of the sucrose-ingesting rats. However, this pattern was not seen in rats that were fed cocaine. The study therefore concluded that the self-administration of cocaine resulted in the “loss of neural integration” in the OFC, thus inhibiting the rat’s ability to use insight and predict outcomes to guide their responding behaviour [17].
Demographics
<style justify>
According to the national survey on Drug Use and Health, the demographic study of cocaine use for the United States in 2014 shows that adults aged 18 to 25 years have a higher rate of current cocaine use than any other age group, with 1.4 percent of young adults reporting past-month cocaine use.[18] There is a higher degree of male cocaine users, and significantly more male overdose cases reported. In 2014 alone, over 5000 cocaine related deaths were reported. [19] White non-hispanics are the most prevalent racial sub-group of cocaine users, followed by blacks, and then hispanics. Internationally, cocaine use is seen highest in North and South America. [20]
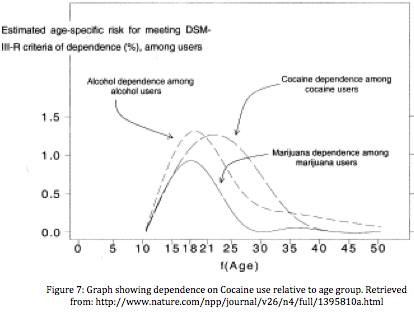
The risk for becoming dependant in the first two years of use is 5-6%, while becoming dependant within 10 years of first use increases to 15-16%. Studies show that the chances of becoming dependant on cocaine was lower when individuals snorted cocaine; higher when smoking, and much higher for injecting. Women are 3.3 times more likely to become dependent when compared to men. Those who started at a younger age, 12-13, were four times more likely to become dependent when compared to those who started at ages 18-20. The peak age for dependence is at ages 23-25. [21]
</style>
Treatment
Pharmacological Treatment
Presently there are not any medications that are approved for formal treatment of cocaine addiction. Many medications have been proven to be ineffective while others showed limited evidence of seeing improvement in cocaine abuse relief. Ibogaine is a pharmacological drug that has been under vigorous investigation recently, and is used in Canadian clinics as a treatment for cocaine use.Other medications used and currently under research include Gabapentin, which makes cocaine cravings easier to overcome and relapses less severe. It increases the neurotransmitter, GABA, which increases relaxation and reduces stress and anxiety. Vigabatrin is another drug that reduces GABA in the brain and reduces cocaine cravings. Baclofen which is still under vigorous research is a muscle relaxant that helps curb cocaine cravings, especially for chronic heavy users. Nocaine provides a weaker version of cocaine's effects, however it is only used by participating research trials. Each drug has its own set of side effects and availability, with most undergoing continuous research. [22]
Behavioural Intervention
Behavioural intervention as treatment for cocaine addiction has shown promising results. The two most common behavioural intervention therapies are cognitive behavioural therapy and motivational therapy. Motivation therapy uses a system that rewards patients that abstain from cocaine use. The patient earns chips or points that can be exchanged for items that can bring positive energy into the cocaine users life, such as gym membership, movie tickets, or dinner vouchers. Cognitive-behavioral therapy is an approach often used to prevent relapse. This helps patients develop the skill needed for long-term abstinence. This therapy helps cocaine users identify and avoid situations where they are most likely to use cocaine and cope more effectively with a variety of issues individuals may experience with drug use. Therapeutic communities are drug free housing residences where people recovery for substance use. Usually therapeutic communities require a 6-12 month stay often include service that help re-integrate individuals into society. The most effective approach is the integration of therapy and pharmacological treatments used together. [19]
Conclusion
Cocaine is a highly addictive and illicit drug. It has been used and abused by humans for thousands of year. It affects the brain by inhibiting GABAergic neurons and thus causes over-activation of the body’s natural reward system, often leading to recurring use and addiction. Cocaine has negative effects on all aspect of the body including the lungs, liver, kidneys and sexual functioning. In addition, the drug can permanently alter various areas of the brain including, the nucleus accumbens, the cerebellum and the orbitofrontal cortex. Although cocaine use has been on the decline since the 1990s, it is still one of the most prevalent drugs in North America today and poses problems in regards to addiction, overdoses and illegal drug trade. Future research on cocaine is focused on developing and refining pharmacological drugs that can be implicated in treating cocaine abuse and addiction. In addition, research and clinical trials are being conducted in regards to anti-cocaine vaccines that may be able to block the effects of cocaine and effectively treat cocaine addiction.
References
- Biondich, A. S., Joslin, J. D. (2016). Coca: the History and Medical Significance of an Ancient Andean Tradition. Emergency Medicine International, 2016, 1-5. doi: 10.1155/2016/4048764
- National Institute on Drug Abuse. (n.d.). Cocaine. Retrieved from https://www.drugabuse.gov/publications/drugfacts/cocaine
- Johnston, A. J., Busch, S., Pardo, L. C., Callear, S. K., McLain, S. E. (2016). On the atomic structure of cocaine in solution. Physical Chemistry Chemical Physics, 18, 991- 999. doi: 10.1039/c5cp06090g.
- Methoide. (n.d.). Cocaine: Pharmacology. Retrieved from http://methoide.fcm.arizona.edu/infocenter/index.cfm?stid=168
- Stewart, D. J., Inaba, T., Lucassen, M., Kalow, W. (1979). Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clinical Pharamcology and Therapeutics, 25, 464-468.
- Nestler, E. (2005). The Neurobiology of Cocaine Addiction. Science & Practice Perspectives, 3(1), 4-10. http://dx.doi.org/10.1151/spp05314 Center for Behavioral Health Statistics and Quality. (2015).
- Goldberg, J. (2016). Cocaine Use and Its Effects. WebMD. Retrieved 23 September 2016, from http://www.webmd.com/mental-health/addiction/cocaine-use-and-its- effects?page=3
- Nicholls, J.G., Mar5n, A.R., Wallace, B.G. & Fuchs, P.A. (2001) From Neuron to Brain, 4th Ed.
- Breiter, Hans C., et al. “Acute effects of cocaine on human brain activity and emotion.” Neuron 19.3 (1997): 591-611.
- Mackey, S., & Paulus, M. (2013, March). Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neuroscience & Biobehavioral Reviews, 37(3), 300-316. doi:10.1016/j.neubiorev.2012.12.003
- Dubuc, B. (n.d.). THE BRAIN FROM TOP TO BOTTOM. Retrieved September 21, 2016, from http://thebrain.mcgill.ca/flash/i/i_03/i_03_cr/i_03_cr_par/i_03_cr_par.html
- Robinson, T. E., Gorny, G., Mitton, E., & Kolb, B. (2001). Cocaine self‐administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens- and neocortex. Synapse, 39(3), 257-266. doi:10.1002/1098 2396(20010301)39:33.3.co;2-t
- Porter, J. N., Minhas, D., Lopresti, B. J., Price, J. C., & Bradberry, C. W. (2014). Altered cerebellar and prefrontal cortex function in rhesus monkeys that previously self- administered cocaine. Psychopharmacology, 231(21), 4211-4218. doi:10.1007/s00213-014-3560-
- Paulin, M.G. (1993). The Role of the Cerebellum in Motor Control and Perception. Brain Behav Evol, 41(1), 39-50 1993. http://www.otago.ac.nz/neurozoo/documents/Paulin%201993%20-%20role%20of%20the%20cerebellum.pdf
- Bechara, A. (2000). Emotion, Decision Making and the Orbitofrontal Cortex. Cerebral Cortex, 10(3), 295-307. doi:10.1093/cercor/10.3.295
- Hester, R. (2004). Executive Dysfunction in Cocaine Addiction: Evidence for Discordant Frontal, Cingulate, and Cerebellar Activity. Journal of Neuroscience, 24(49), 11017-11022. doi:10.1523/jneurosci.3321-04.2004
- Lucantonio, F., Takahashi, Y. K., Hoffman, A. F., Chang, C., Bali-Chaudhary, S., Shaham, Y., Schoenbaum, G. (2014). Erratum: Orbitofrontal activation restores insight lost after cocaine use. Nature Neuroscience Nat Neurosci, 17(9), 1287-1287. doi:10.1038/nn0914-1287e
- Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/ data/
- What is the scope of cocaine use in the United States? (2016). Drugabuse.gov. Retrieved 23 September 2016, from https://www.drugabuse.gov/publications/research-reports/cocaine/what-scope-cocaine-use-in-united-states
- Cooper, L. (2003) (2nd ed.). Maryland: National Institute of Drug Abuse. Retrieved from https://archives.drugabuse.gov/pdf/minorities03.pdf
- Wagner, F. (2002). From First Drug Use to Drug Dependence Developmental Periods of Risk for Dependence upon Marijuana, Cocaine, and Alcohol. Neuropsychopharmacology, 26(4), 479-488. http://dx.doi.org/10.1016/s0893 133x(01)00367-0
- Cocaine : Addiction Medication : The Addiction Recovery Guide. (2016). Addictionrecoveryguide.org. Retrieved 23 September 2016, from http://www.addictionrecoveryguide.org/medication/cocaine