This is an old revision of the document!
Pathophysiology
The limbic brain regions associated with mood and depression include the hippocampus and the prefrontal cortex. Studies suggest that stress and depression are associated with atrophy and loss of neurons and glia, which contribute to the decreased size and function of these brain areas.
The Monoamine-deficiency hypothesis: The first real indication that depression might result from a problem with the central nervous system modulatory systems came in the 1960s. A drug called reserpine, introduced to control high blood pressure, caused psychotic depression in about 20% of cases. Its mechanism of action involves the depletion of central catecholamines and serotonin by interfering with their loading into synaptic vesicles. Another class of drugs introduced to treat tuberculosis caused a marked elevation in mood. These drugs inhibit monoamine oxidase (MAO) which is the enzyme that degrades catecholamines and serotonin in the synaptic cleft. Furthermore, when it was discovered that the drug imipramine, introduced as an antidepressant years earlier, inhibits the reuptake of released serotonin and norepinephrine, thus promoting their action in the synaptic cleft. As a result of these observations, researchers developed the hypothesis that mood is closely tied to the levels of released monoamine neurotransmitters, norepinephrine and/or serotonin, in the brain. According to this monoamine hypothesis, depression is a consequence of a deficit in one of these diffuse modulatory systems.
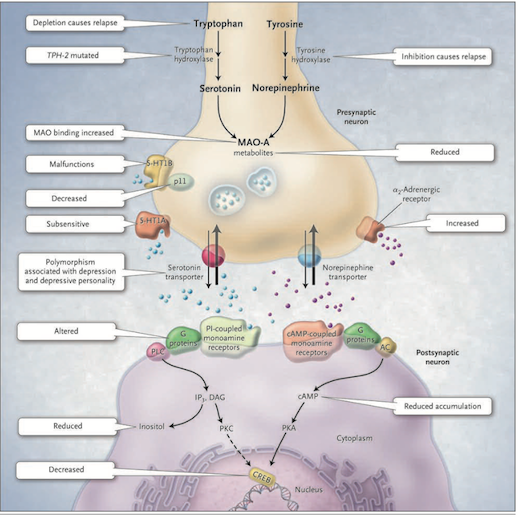
Figure 1: The Monoamine-deficiency hypothesis (Source: Bedmaker & Agam, 2008) </style>
This hypothesis postulates a deficiency in serotonin or norepinephrine neurotransmission in the brain. Serotonin is synthesized from tryptophan by the enzyme tryptophan hydroxylase; norepinephrine is synthesized from tyrosine which is catalyzed by the enzyme tyrosine hydroxylase. Both monoamine transmitters are stored in vesicles in the presynaptic neuron and released into the synaptic cleft, thereby affecting both presynaptic and postsynaptic neutrons. Neurotransmission is mediated by serotonin (5-hydroxytryptamine 1A [5-HT1A] and 5-hydroxytryptamine 1B [5-HT1B]) or norepinephrine receptors. There are several mechanisms by which the activity of these neurotransmitters can be terminated. One possibility is through reuptake through the specific serotonin and norepinephrine transporters and by feedback control of release through the presynaptic 5-HT1A and 5-HT1B regulatory autoreceptors for serotonin and the α2-noradrenergic autoreceptors for norepinephrine. Monoamine oxidase A (MAO-A) catabolizes monoamines presynaptically and thereby indirectly regulates vesicular content. The function of the 5-HT1B receptor is increased by the p11 protein and therefore a decrease in this p11 protein is thought to be implicated in depression. On the postsynaptic neutron, serotonin and norepinephrine bind G-protein coupled receptors including the cyclic AMP (cAMP)-coupled receptor and the phosphatidylinostiol-coupled receptor. The downstream effects of the cAMP-coupled receptor are to activate adenylyl cyclase which generates cAMP which in turn activates protein kinase A (PKA). The phosphatidylinostiol pathway activates phospholipase C which generates two second messengers, IP3 (activates Ca2+ release) and diacylglycerol (activated protein kinase C (PKC)). The two protein kinases affect the cAMP response element–binding protein (CREB). Findings in patients with depression that support the monoamine-deficiency hypothesis include a relapse of depression with inhibition of tyrosine hydroxylase or depletion of dietary tryptophan, an increased frequency of a mutation affecting the brain-specific form of tryptophan hydroxylase (TPH-2), increased specific ligand binding to MAO-A, subsensitive 5-HT1A receptors, malfunctioning 5-HT1B receptors, decreased levels of p11, polymorphisms of the serotonin-reuptake transporter associated with depression, an inadequate response of G proteins to neurotransmitter signals, and reduced levels of cAMP, inositol, and CREB in postmortem brains [Figure 1].
The Diathesis-stress hypothesis: According to this hypothesis, the hypothalamic-pituitary-adrenal (HPA) axis is the main site where genetic and environmental influences converge to cause mood disorders. As mentioned earlier, depression can run in families and our genes can predispose us to mental illness. Furthermore, early childhood abuse or neglect, and other stresses of life, are important risk factors in the development of depression in adults. Hyperactivity of the HPA axis results in elevated blood cortisol levels as well as higher concentrations of corticotropin-releasing hormone in the cerebrospinal fluid. Injected CRH into the brains of animals produces behavioural effects that are similar to those of major depression: insomnia, decreased appetite, decreased interest in sex and increased behavioural expression of anxiety.
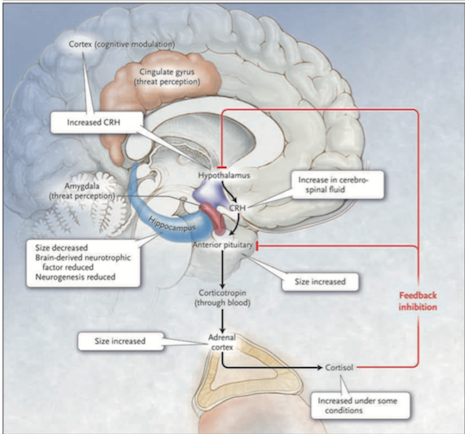
Figure 2: The HPA system in depression (Source: Bedmaker & Adam, 2008) </style> Abnormalities in the cortisol response to stress may underlie depression. The black arrows in Figure 2 show that in response to stress, which is perceived by the brain cortex and the amygdala and transmitted to the hypothalamus, corticotropin-releasing hormone (CRH) is released. This induces the anterior pituitary gland to secrete corticotropin into the bloodstream. Corticotropin stimulates the adrenal cortexes to secrete the glucocorticoid hormone cortisol. The red lines Figure 2 show that cortisol, in turn, induces feedback inhibition in the hypothalamus and the pituitary, suppressing the production of CRH and corticotropin, respectively. Activation of hippocampal glucocorticoid receptors by cortisol normally leads to feedback inhibition of the HPA axis. In depressed patients this feedback is disrupted thus leading to the hyperactivation of HPA. A molecular basis for the diminished hippocampal response to cortisol is a decreased number of glucocorticoid receptors. The number of receptors is regulated by genes, monoamines and early childhood experiences. Findings in patients with depression that support the hypothalamic–pituitary–cortisol hypothesis include the following: cortisol levels are sometimes increased in severe depression, the size of the anterior pituitary and adrenal cortex is increased, and CRH levels in the cerebrospinal fluid and CRH expression in the limbic brain regions are increased. Hippocampal size and the numbers of neurons and glia are decreased, possibly reflecting reduced neurogenesis due to elevated cortisol levels or due to reduced brain-derived neurotrophic factor.