Table of Contents
Ascariasis
What is Ascariasis?
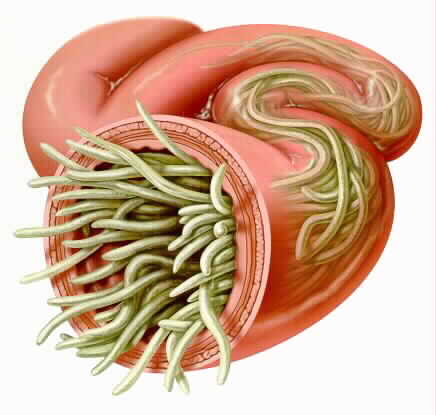
One of the most prevalent yet neglected tropical diseases worldwide with 800 million-1.2 billion annual cases (Shah & Shahidullah, 2018). Ascariasis is caused by roundworms that usually target the intestines. Similar to other parasites, they develop within the host from larvae or egg to a mature adult-worm. The parasites that achieve biological maturity and are capable of reproducing are typically 12 inches or 30cm in length (Mayo Clinic, 2018).
History
The presence of ascariasis can date back to 2277 BC, where A. lumbricoides eggs were discovered in the fossilized feces of a human from Peru (Cox, 2002). Ascariasis is an ancient disease with records dating back to 1938 and 1600 BC of A. lumbricoides in a Middle Kingdom Egyptian mummy and from the Ming Dynasty in China between AD 1368 and 1644 (Cox, 2002).
The first detailed anatomy of A. lumbricoides was depicted in the late 17th century by an English physician - Edward Tyson (Cox, 2002). Soon after, Francesco Redi wrote a book describing the worm; it was one of the first books of parasitology (Cox, 2002). The study of helminthology reached its peak in the 19th century, partly due to these two publications (Cox, 2002). Scientist began attempting to understand the infections caused by Ascaris and how to treat them (Cox, 2002).
In 1878, Italian physician and zoologist, Giovanni Battista Grassi, was the first to demonstrate the infectivity of A. lumbricoides by ingesting the eggs found in the feces of those who died from the disease (Cox, 2002; Silva, 2019). It wasn’t until 1922 when a Japanese pediatrician, Shimesu Koino, infected both a volunteer and himself, that he discovered the life cycle of Ascaris in the human body (Cox, 2002).
Acaris subtypes
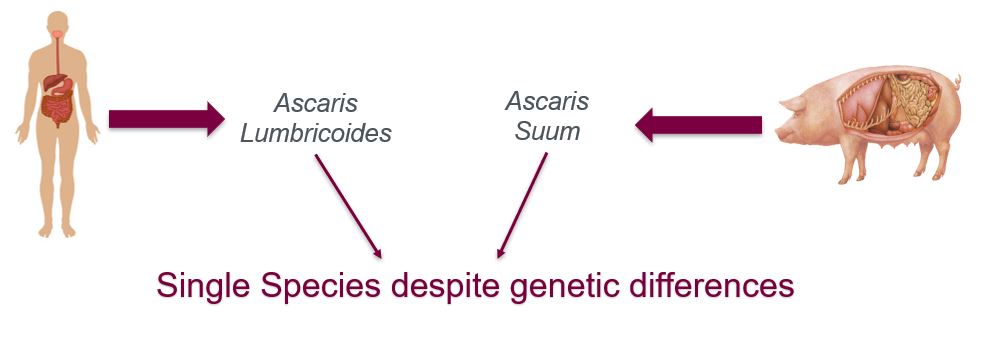
Ascaris Lumbricoides typically infects human populations whereas Ascaris suum is most commonly observed in animal populations. The two parasites are also referred to as geo-helminths suggesting that the infection is primarily transmitted via contaminated soil (Center for Control and Disease [CDC], n.d.). Ascaris eggs in the soil are capable of surviving up to 10 years, and only take 10-15 days to become infective in the soil (CDC, n.d.)
The two roundworms are drastically different genetically, in fact in the past it has been debated whether they should be classified as two different species. However, recently following a molecular analysis extreme similarities in the worms’ nucleotide and amino acid sequences have led researchers to classify them as a single species (Monteiro et al., 2019). Additionally, both roundworms have been observed to infect humans and pigs supporting the notion that despite certain genetic differences the two worms are the same species. Furthermore, they’re almost indistinguishable morphologically and follow a similar infectious pathway once inside the host. The subtle morphological differences such as the shape of lips and size of teeth can be detected through an Electron microscope (Monteiro et al., 2019).
The worms are both anthroponotic and zoonotic meaning that cross-transmission of the infection is bi-directional. Humans and pigs are both capable of transmitting the infection to each other (Monteiro et al., 2019).
Statistics
Did you know that ~120-220 million cases are symptomatic and ~1.2 billion cases occurred annually.
Epidemiology,Prevalence and Risk
Ascariasis lumbricoides is a highly prevalent intestinal worm diseases which has major risks and consequences worldwide (Beaver, 1952). It is usually associated and is found more prevalent in tropical and subtropical areas that host a generally warmer climate regime (Bethony et al., 2006). Although the infection manifests in both developed and developing countries such as Asia and sub-Saharan Africa, it is more frequent in the latter simply due to poverty, governmental ignorance and corruption, poor hygiene and sanitary living conditions (Cort, 1931). Moreover, the infection is pervasive in areas where farming practices incorporate the use of feces as fertilizer and has a high correlation in areas occupied with pigs (Beaver, 1952). The disease represents itself as a global health challenge as children, that are the most susceptible to this infection, can experience significant neurological deficits upon diagnosis (Cort, 1931). These may include, but are not limited to, cognitive impairment, intestinal and stomach complications, and death (Cort, 1931).
Recent research also indicates that the global disability adjusted life years lost from Ascaris is somewhere around 10.5 million (Cort, 1931). The species can also infestate pigs and cause symptoms that are similar in humans, including decreased food intake, malabsorption, nitrogen loss and hindered absorption of essential vitamins (Beaver, 1952). When it comes to the global burden of disease, about 120-220 million cases are symptomatic and approximately 1.2 billion cases have occurred already (Das, 2014). Almost 60,000 deaths occur every year, most of whom are children under the age of 12 (Bethony et al., 2006). In addition, in numerous endemic regions, infants are usually exposed to risk of infection from birth and are simultaneously at risk for reinfection after appropriate treatment and recovery (Bethony et al., 2006).
Life Cycle
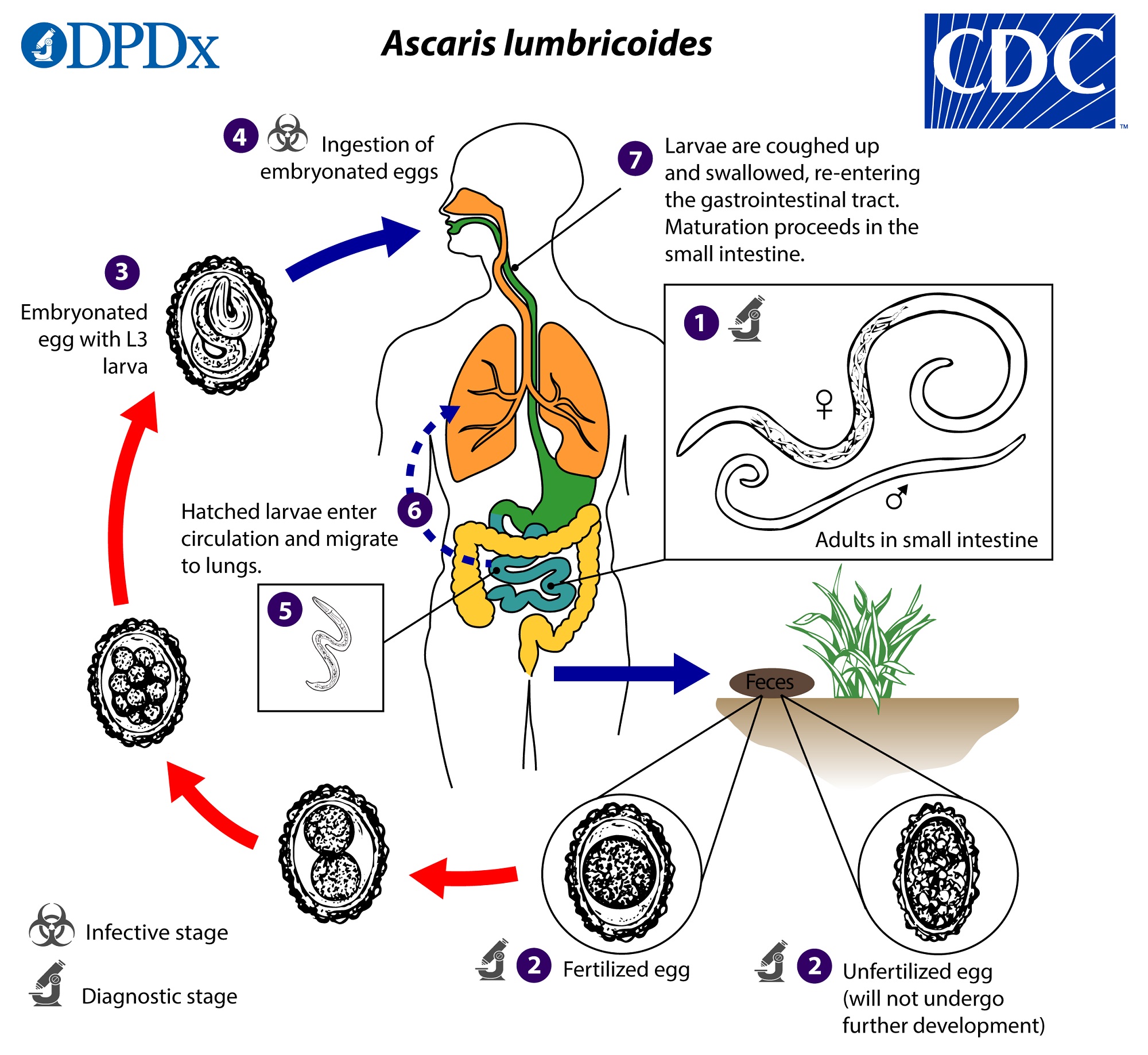
Ascaris infections are contracted via the oral route, often from ingesting infective A. lumbricoide eggs (Murrell et al., 1997). A female adult worm can produce approximately 200,000 eggs per day, which are then passed with the feces of the host (CDC - Ascariasis - Biology, 2019).These eggs can be non-infective if the eggs are unfertilized, however larvae within fertile eggs will develop to infectivity after 18 days to several weeks (CDC - Ascariasis - Biology, 2019). Although, how quickly they develop to infectivity is highly dependent on its environmental conditions; with the optimum environment being warm and moist with shaded soil (CDC - Ascariasis - Biology, 2019). Following the ingestion of an infective egg, it will hatch in the small intestine and migrate to the lungs through the portal, then systemic circulation (CDC - Ascariasis - Biology, 2019). Once the larvae is at the lungs, it will mature for 10 to 14 days before penetrating the alveolar walls and ascending the bronchial tree to reach the throat before being swallowed once more (CDC - Ascariasis - Biology, 2019). Hence, the larvae returns to the small intestine where it will develop into adult worms (CDC - Ascariasis - Biology, 2019). Female adult worms take 2 to 3 months from ingestion of the infective eggs to produce more eggs, while the male adult worms can live one to two years within its host (Mayo Clinic Staff, 2018).
Clinical Manifestation
What are the symptoms?
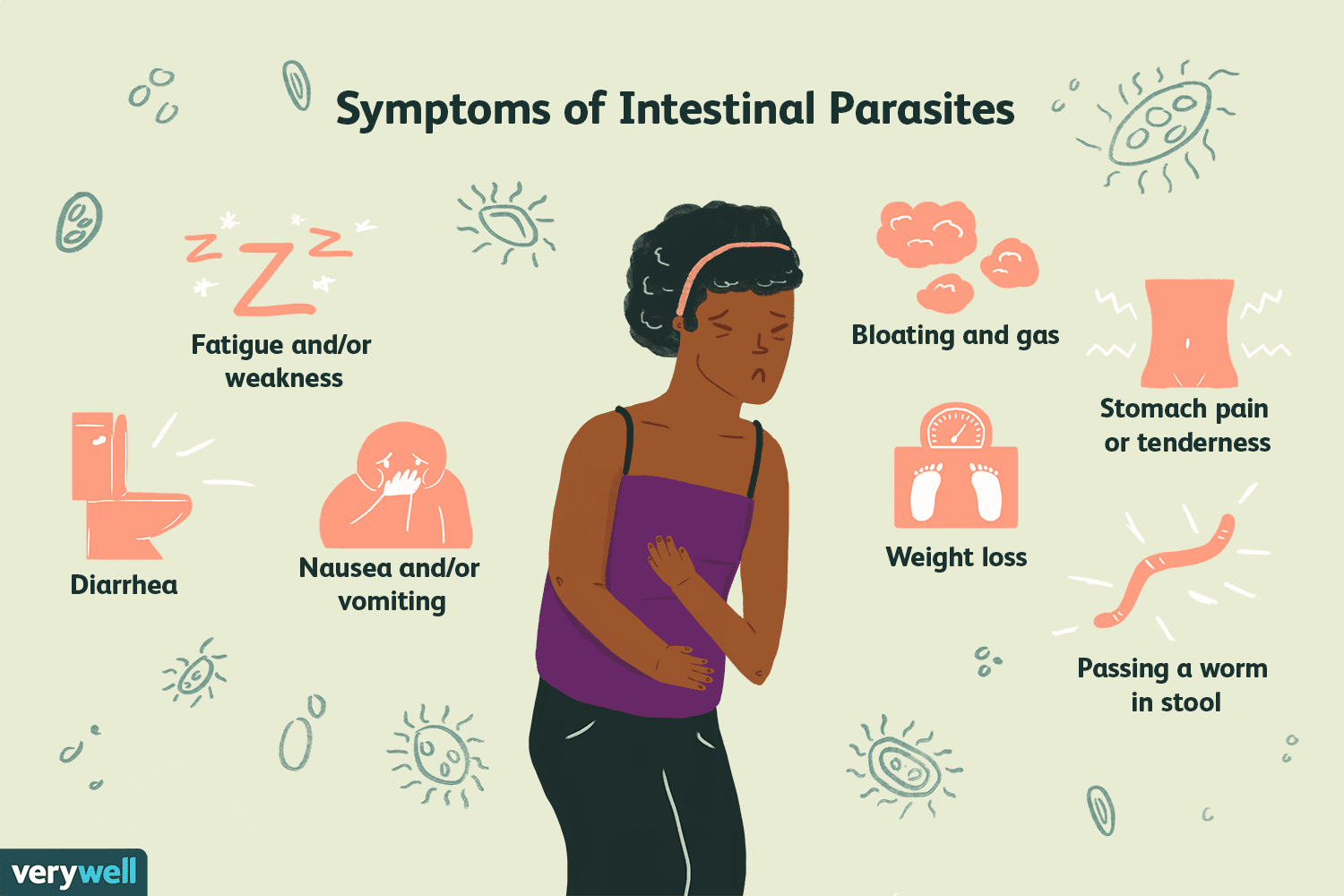
Majority of individuals infected with Ascaris often show no symptoms (CDC- Ascariasis-Biology, 2019). However sometimes the following symptoms are shown: abdominal discomfort, swelling, pain, nausea, vomiting, fever, coughing, wheezing and passage of parasites and their eggs in stools (Ben-Joseph, 2019; Dold & Holland, 2011). Symptoms have been shown to increase with the increase of number of worms. There is a positive proportional relationship between symptoms and number of eggs (Dold & Holland, 2011).
Note: Ascariasis is not contagious, in order to become infected a person must swallow the worm’s eggs.
When matters are left unresolved, it could lead to migrating larvae, intestinal blockage, bowel obstruction, malnutrition and in some cases death.
Etiology
Ascaris lumbricoides which is a soil transmitted roundworm causes ascariasis (Lima & Horrall, 2019). The adult male can reach up to 15-20 cm in length while the adult female reaches up to 20-30 cm (Lima & Horrall, 2019).Female worms are also thicker compared to male worms (Lima & Horrall, 2019). On average, an adult parasite lives for a year. After parasite dies, it leaves the digestive tract (Lima & Horrall, 2019).A person can become infected with ascariasis after accidentally ingesting A. lumbricoides eggs (Ascariasis, 2019). This infection can also occur through eating or drinking contaminated food or water, especially if the individual did not wash the food and his/her hands (Fletcher, 2018). For instance, an individual can contract ascariasis from the consumption of uncooked raw vegetables grown in soil contaminated by roundworm eggs (Ascariasis, 2019). These eggs are usually found in soil that is contaminated with human feces (Ascariasis, 2019). Finally, children are often infected after playing in contaminated soil and putting their hands in their mouth (Fletcher, 2018).
Pathophysiology
The pathophysiology of ascariasis is linked to considerable organ damage (Das, 2014). The larvae entering into the intestinal mucosa and the liver leads to hypersensitivity and increased immune response (Kakihara et al., 2004). Upon the response and activation and attachment of eosinophils to the larvae itself, some may be immobilized and restricted for further movement (Kakihara et al., 2004). The stoppage and restriction of many larvae can cause the formation of aggregation of granulomas, a collection of macrophages that have the capacity to calcify and harden (Khuroo, 1996).
In lungs, the transportation of the larvae from the vessels into the bronchioles can lead to haemorrhage that results in inflammation and swelling of the alveoli (de Silva, Chan & Bundy, 1997). Specifically, it causes alveolar sacs to be filled by a serous exudate causing the release of eosinophils and basophils that further induces goblet cell function and mucus formation in the bronchi (de Silva, Chan & Bundy, 1997). In clinical terms, this is known as Loeffler's syndrome which includes symptoms such as dry cough, and high fever (de Silva, Chan & Bundy, 1997). The impact is directly proportional to the number of worms reaching the intestinal mucosa in the first place (Khuroo, 1996).
The transmission of the worms from the stomach to the intestines causes varied changes in the jejunum and intestinal epithelial layers (Das, 2014). Specifically, the attachment of worms to the intestinal muscle and epithelium causes a series of reactions that result in a decrease of the crypt death, loosening of the mucosal folds and limited food absorption (Beaver, 1952). Scientists state that the effect of the aforementioned pathogenesis is what leads to the neurological deficits in children and is linked to protein energy malnutrition (Kakihara et al., 2004). Something that is especially common in adult Ascaris is the obstruction of the intestinal and pancreatic duct pathway due to their sheer size and their ability to aggregate and accumulate at various entry points throughout the human digestive system (Holmes, 1987). This may be accompanied by conditions involving acute pancreatitis, eosinophilia, cholecystitis and hypertrophy of the intestinal tissue layers (Holmes, 1987).
Consequently, some larvae and worms may be killed by the activation of various immune response T cells (Das, 2014). This, however, is not always good news because our body has an increasingly difficult time to excrete them through feces and other excretory mechanisms (Khuroo, 1996). Dead Ascaris can cause gallstone formation in the bile duct to the inflammatory molecules present in the worm cuticle (Khuroo, 1996). This is supported by a study in which worm debris have been located in bilirubin calcium stones in individuals affected with ascariasis (Kakihara et al., 2004).
Prevention and Control
In areas of frequent and intense periods of infections, mass drug administration (MDA) is conducted annually under national supervision in specific communities at higher risk of being affected (WHO, 2006). Drugs are administered in predetermined doses according to the age of an individual. This practice of MDA which helps prevent long-term adverse effects of worm-related diseases is referred to as preventive chemotherapy. MDA can be conducted in various ways for example, distributors can go door-to-door, set up booths near target communities or in areas of public gathering (i.e. railways, marketplaces, festivals) or pay special visits to certain institutions (e.g. schools, hospitals, refugee camps) (WHO, 2006). Preventive chemotherapy has shown to help control infections, reduce transmissions and prevent morbidity in those already infected. A drug can be administered alone, or in combination with other anthelmintics which provides the benefit of targeting several worm-related infections simultaneously. Thus, preventive chemotherapy offers a safe and inexpensive method for preventing and controlling outbreaks (WHO, 2006)
Additional simple ways of preventing and controlling infection is to increase health literacy among the population in order to promote good hygiene and improved sanitary practices. Public should be cautioned against defecating outside to prevent soil contamination. Authorities should facilitate improved sewage and sanitary systems especially in poor areas to avoid contact with fecal matter to prevent disease transmission. Reducing the use of human and pig feces for crop fertilization is another important precautionary measure that can be taken to avoid the proliferation of the disease (CDC, 2018). Ensure proper hand washing before and after handling food items. Wash raw vegetables and fruits thoroughly before ingesting. The safest method for consuming foods grown in contaminated soil is to peel the skin off and cook them well before consumption. While travelling or during periods of increased infection, avoid drinking unfiltered water as it could be contaminated with worm eggs.
Since, the infection can also be transmitted via animals, particularly the swine population, similar precautions should be practiced while interacting with them. Ensure your hands are clean before and after coming into contact with pigs. Children should be especially taught good hygienic and sanitary practices and supervised when playing outside in the soil or while interacting with farm animals (CDC, 2018).
Diagnosis
Tests and Examinations
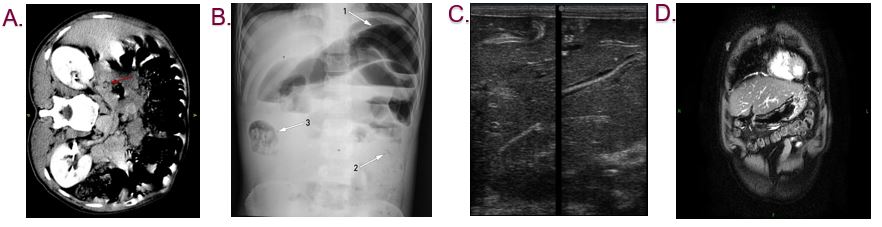
Adult female ascariasis worms lay eggs in the intestine (Ascariasis, 2018). These eggs then travel through the digestive system and can eventually be found in the stool (Ascariasis, 2018). Eggs take at least 40 days to appear in the stool sample after an individual is infected. If the individual is infected with male worms, then there would be no eggs (Ascariasis, 2018). Diagnosis usually happens by a doctor through examining a stool sample for parasites and eggs. Stool sample is analyzed under a microscope to identify ascaris eggs (CDC, 2018). It might be hard to find eggs in light infections. Therefore, a concentration procedure is recommended (CDC, 2018). Concentration procedure increases the chance of identifying ascaris eggs even when eggs are present in small numbers (CDC, 2016). This occurs by separating parasites from fecal debris (CDC, 2016). If the person is diagnosed with this disease then an imaging tests such as x-ray, ultrasound, CT scan, or MRI might be required (Ascariasis, 2019). To begin with, x-rays are electromagnetic radiations that produce images of the structures inside the body (Lucas, 2018). X-ray can show the worm’s mass in the abdomen. Also, a chest x-ray can identify the larvae in the lungs (Ascariasis, 2018). Ultrasound uses high frequency soundwaves to view internal organs. This imaging technique can show worms in the liver or pancreas (Ascariasis, 2018). CT scans and MRI can detect worms that are blocking ducts in the pancreas or liver (Ascariasis, 2018). These tests can show the doctor the location of worms inside the body and also the number of worms that have reached maturity (Ascariasis, 2019). Finally, in some cases of heavy infestation, it is possible for worms to come out of nostrils or mouth after coughing or vomiting (Ascariasis, 2018). In this situation it is recommended to take the worm to a doctor so the doctor can identify the worm and prescribe a treatment (Ascariasis, 2018).
Figure 6. Different imaging techniques showing ascariasis. A. CT scan B.X-ray C. Ultrasound D. MRI. Retrieved from https://prod-images-static.radiopaedia.org/images/9266208/0674081a3cd727a37588ee4b3160ed_gallery.jpg; Retrieved from https://radiopaedia.org/articles/biliary-ascariasis?lang=us; Retrieved from https://radiopaedia.org/articles/biliary-ascariasis?lang=us; Retrieved from https://ak1.picdn.net/shutterstock/videos/26476841/thumb/1.jpg .
Current Treatment and Therapeutics
Medication
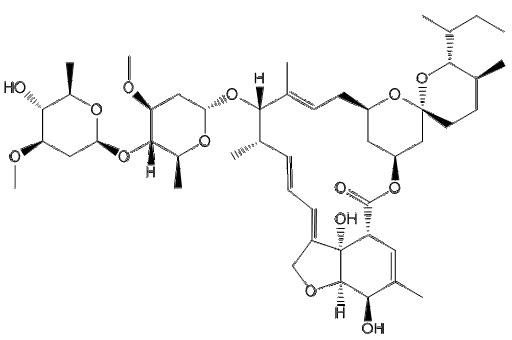
Medication used to treat roundworms are known as acaricides. Those recommended by the Centers for Disease Control and prevention (CDC) include: Albendazole, Mebendazole, and Invermectin (CDC-Ascariasis, 2019). Another possible therapeutic is Pyrantel (Silva, R.D., 2019).
1. Albendazole and Mebedazole
Albendazole and Mebendazole belong to the family of medicines called anthelmintics. They are used to treat a variety of worm infections, including ascariasis. These medications work by interrupting the cytoplasmic microtubules in the intestinal cells of the worms, preventing the movement and polymerization of microtubules (Silva, R.D., 2019). Hence, it prevents the worm from absorbing glucose, causing the worm to have difficulty moving (Silva, R.D., 2019). As a result, the worm is depleted of its glycogen storage and starves to death (Silva, R.D., 2019). Albendazole should be taken at a 400mg dosage, once orally, while Mebendazole should be taken at a 100mg dosage, orally twice daily for 3 days or at a dosage of 500mg, once orally (CDC - Ascariasis, 2019). However, this medication is poorly absorbed in the host’s intestinal tract (Silva, R.D., 2919). The video shown below is an animation that represents how Albendazole and Mebendazole work.
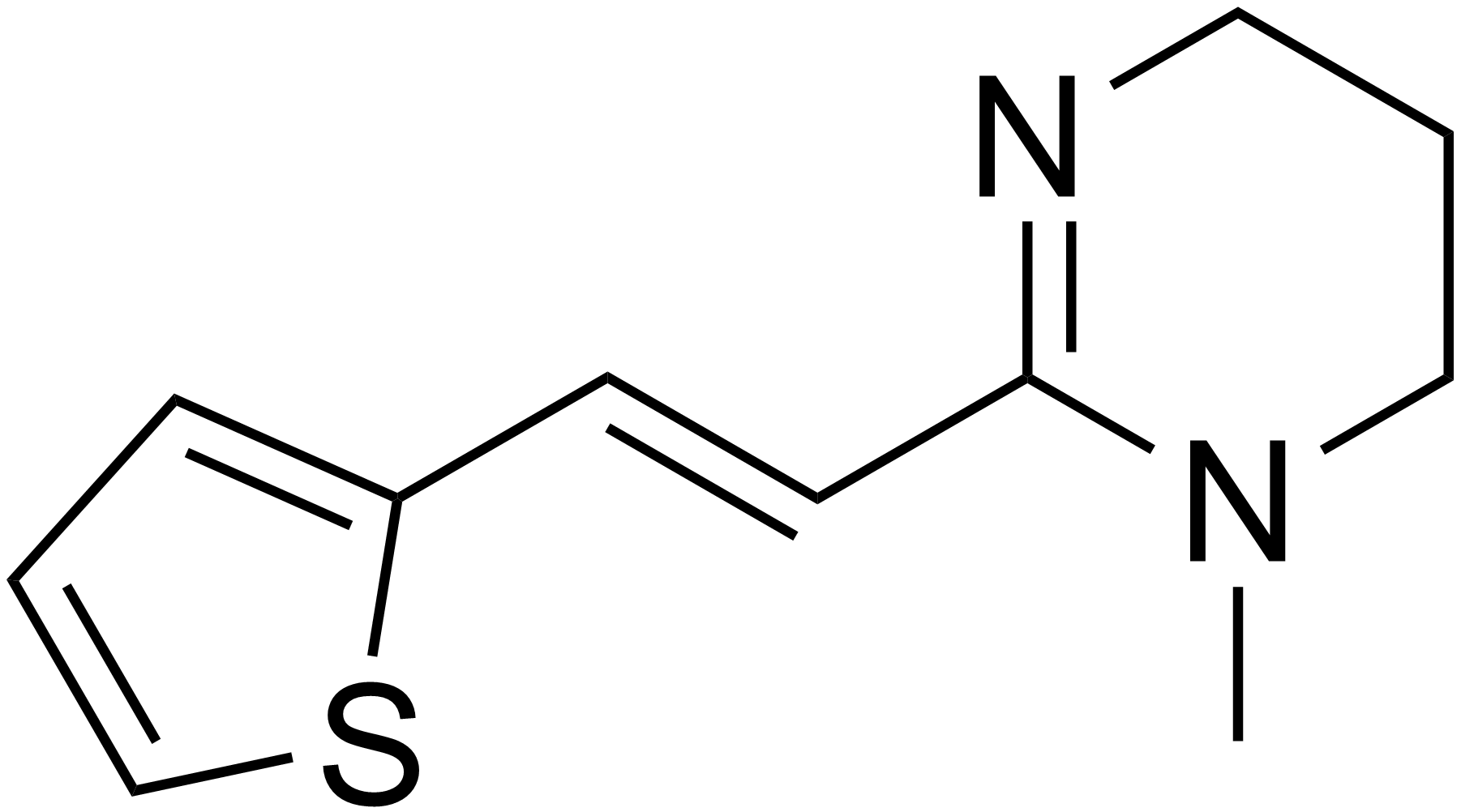
2. Ivermectin
Ivermectin is a macrocyclic lactone medicine also used to treat parasitic infections (Smith, 2014). Ivermectin stimulates an excessive release of neurotransmitters in the peripheral nervous system of the parasite causing the parasite to reside in a state of paralysis or inactivating its gut (Smith, 2014). This medication can also act on the human body, targeting the brain (Smith, 2014). However, the blood-brain barrier of the brain protects ivermectin from reaching (Smith, 2014). Ivermectin should be taken once orally at a dosage of 150 to 200mcg/kg (CDC - Ascariasis, 2019).
3. Pyrantel
Pyrantel is a nicotinic acetylcholine receptor agonist (Silva, R.D., 2019). This type of medication acts by causing spastic muscle paralysis in the parasitic worms (Silva, R.D., 2019). This is a result of a constant release of acetylcholine in the body wall muscle of the parasitic worms (Silva, R.D., 2019). Hence, the worm becomes incapable of moving, allowing the body to sweep the worm out naturally through the host’s stool (Silva, R.D., 2019). However, this medication is also poorly absorbed in the host’s intestinal tract (Silva, R.D., 2019).
Surgery
In the case of heavy infestation, surgery may be required to remove large amounts of worms and to repair the damages that they have caused. According to Mayo Clinic, some complications that require surgery include: intestinal obstruction or perforation, appendicitis, and bile duct obstruction (Mayo Clinic Staff, 2018). During surgery, the worms may be manually removed by massaging the worms out the intestine through a cut opening (Silva, R.D., 2019).
References
Ascariasis. (2018, May 17). Retrieved January 22, 2020, from https://www.mayoclinic.org/diseases-conditions/ascariasis/diagnosis-treatment/drc-20369597
Ascariasis: Causes, Symptoms, and Treatments. (2019, April 17). Retrieved from https://www.healthline.com/health/ascariasis
Ben-Joseph, E. P. (Ed.). (2019, November). Ascariasis (for Parents) - Nemours KidsHealth. Retrieved from https://kidshealth.org/en/parents/ascariasis.html
CDC - Ascariasis - Biology. (2019, July 19). Retrieved January 22, 2020, from https://www.cdc.gov/parasites/ascariasis/biology.html
CDC - Ascariasis - Diagnosis. (2018, February 15). Retrieved from https://www.cdc.gov/parasites/ascariasis/diagnosis.html
CDC - DPDx - Diagnostic Procedures - Stool Specimens. (2016, May 3). Retrieved from https://www.cdc.gov/dpdx/diagnosticprocedures/stool/specimenproc.html
CDC - Ascariasis - Resources for Health Professionals. (2019, August 28). Retrieved January 22, 2020, from https://www.cdc.gov/parasites/ascariasis/health_professionals/index.html#tx
Centers for Disease Control and Prevention. (n.d.). Ascariasis. Retrieved from https://www.cdc.gov/parasites/ascariasis/
Cox, F. E. (2002). History of human parasitology. Clinical microbiology reviews, 15(4), 595-612.
Dold, C., & Holland, C. V. (2011). Ascaris and ascariasis. Microbes and Infection, 13(7), 632–637. doi: 10.1016/j.micinf.2010.09.012
Fletcher, J. (2018, July 3). Ascariasis: Causes, symptoms, and treatment. Retrieved from https://www.medicalnewstoday.com/articles/322340.php#causes
Hall A., Hewitt G, Tuffrey V. (2008). A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Maternal and Child Nutrition. 4: 118–236. doi:10.1111/j.1740-8709.2007.00127.x
Lima Corvino, D. F. D., & Horrall, S. (2020). Ascariasis [Jan 12 2019]. Treasure Island , FL: StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK430796/
Lucas, J. (2018, October 5). What Are X-Rays? Retrieved from https://www.livescience.com/32344-what-are-x-rays.html
Mayo Clinic. (May 17, 2018). Ascariasis: Symptoms and Causes. Retrieved from https://www.mayoclinic.org/diseases-conditions/ascariasis/symptoms-causes/syc-20369593
Monteiro, K. J., Calegar, D. A., Santos, J. P., Bacelar, P. A., Coronato-Nunes, B., Reis, E. R. C., … & Jaeger, L. H. (2019). Genetic diversity of Ascaris spp. infecting humans and pigs in distinct Brazilian regions, as revealed by mitochondrial DNA. PloS one, 14(6), e0218867.
Murrell, K. D., Eriksen, L., Nansen, P., Slotved, H. C., & Rasmussen, T. (1997). Ascaris suum: a revision of its early migratory path and implications for human ascariasis. The Journal of parasitology, 255-260.
Shah, J., & Shahidullah, A. (2018). Ascaris lumbricoides: a startling discovery during screening colonoscopy. Case reports in gastroenterology, 12(2), 224-229.
Smith, J. (2014, December). Ivermectin. Retrieved January 22, 2020, from https://www.dermnetnz.org/topics/ivermectin/
Stephenson, L.S. (1987). The Impact of Helminth Infections on Human Nutrition. London: Taylor & Francis.
Török M. Estée., Cooke, F. J., & Moran, E. (2017). Oxford handbook of infectious diseases and microbiology. Oxford: Oxford University Press.
WHO. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Crompton DWT, editor. WHO Library. Geneva, Switzerland: WHO Press; 2006.