This is an old revision of the document!
Table of Contents
History & Anatomy
History
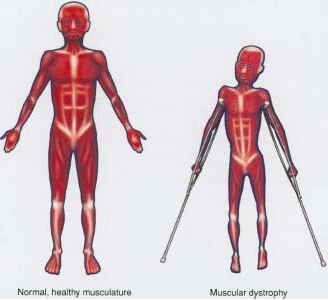
Muscular dystrophy was first noticed in 1830 by Sir Charles Bell who wrote a report about an illness that caused progressive weakness in boys (NIH, 2016). Then in 1836, scientist Conte and Gioja reported two brothers with progressive weakness at at the age of ten (Thompson, 2016). Later these two boys developed generalized weakness and hypertrophy of multiple muscle groups. At that time, many thought that these scientist were describing symptoms of tuberculosis (Thompson, 2016).
In the 1850s, descriptions of boys who experience similar symptoms such as growing progressively weaker, losing the ability to walk, and dying at an early age became more common in medical journals (NIH,2016). It wasn’t until 1868, where French neurologist Guillaume Duchenne gave a comprehensive account of 13 patients with the disease (Cordier et al, 2000). Due to Duchenne high standings for contributing to the understandings of muscle disease one of the most common and classic form of MD is now named after him: Duchenne MD (Cordier et al). After this discovery it became evident that the disease came in different forms and affects all ages and either sex.
Anatomy
DMD is caused by an absence of dystrophin, a protein that helps keeps the muscles of the body intact (Muscular Dystrophy Association, 2016). As a results individuals with MD with experience symmetrical muscle weakness, pseudohypertrophy. Also the muscle is initially edematous but then rapidly becomes atrophic (Muscular Dystrophy Association, 2016).
During the late 1990s, the number of ADHD cases began to significantly increase. This was mainly attributed to the fact that doctors were able to diagnose ADHD more efficiently. Currently, scientists are focusing on identifying the causes of ADHD but we have many medications that treat the disorder with long-term benefits (Lange, Reichl, Lange, Tucha, & Tucha, 2010).
Epidemiology
Incidence rates of MD varies, depending on which MD type is under consideration. Duchenne MD, the most common MD and is sex-linked, has an incidence rate 2 in 1,000 young boys (Human Diseases and Conditions, 2016). The second most common form, Becker MD has an incidence of 1 case per 30,000 live male births (NIH, 2016). The other types of MD are rare. MD affects all races and ethnic groups. It also affects both genders but females are less likely to have MD.
Symptoms
Individuals with Duchenne muscular dystrophy (DMD) are late walkers due to developmental issues. The most prominent sign in individuals with DMD from ages 1 - 3 are enlarged calf muscles, which is known as pseudohypertrophy. DMD children from ages 3 - 5 seem clumsy because they have trouble getting up from the floor, climbing stairs, and fall often. Between the ages of 5 - 10, DMD children usually walk on their toes or heels with a slight waddling gait. Some children try to hold their balance by sticking their stomach out and pulling their shoulders back. It is common for DMD children between the ages 7 - 12 to begin using a wheelchair. By teen year, activities involving gross movement of the arms and legs requires assistance or mechanical support (“Muscular Dystrophy Association”, 2016).
Pain and Sensation
Muscle deterioration caused by DMD is not painful in itself. Individuals on occasion report muscle cramps, which can usually be treated with over-the-counter pain relievers. Since DMD doesn’t directly impact nerves, touch and other senses are normal. In addition, DMD individuals have total control over smooth, involuntary, muscles of the bladder and bowel, and sexual functions (“Muscular Dystrophy Association”, 2016).
The Heart
Individuals with DMD have a lack of dystrophin, which can weaken the myocardium resulting in a condition called, cardiomyopathy. If the damage is very severe during the teen years, it can be life-threatening (“Muscular Dystrophy Association”, 2016).
Respiratory Function
Around the age of 10, the diaphragm and other muscles that operate the lungs begin to weaken, resulting in the lungs being less effective at moving air in and out. Although the individual may not be aware of the weakness, they will complain of shortness of breath, headaches, mental dullness, difficulty concentrating, and nightmares. Weakened respiratory muscles make it difficult for individuals to cough, which increases the risk of serious respiratory infection (“Muscular Dystrophy Association”, 2016).
Learning
Approximately ⅓ of boys with DMD have some degree of a learning disability. Doctors believe that the lack of dystrophin in the brain may have subtle effects on cognition and behaviour. Majority of these learning problems occurs in 3 general areas: attention focusing, verbal learning and memory, and emotion interaction (“Muscular Dystrophy Association”, 2016).
Prognosis & Diagnosis
For any form of muscular dystrophy, a doctor will initially begin by taking a patient's family history, and performing a physical examination before any complicated diagnostic tests are done.
Creatine Kinase Levels
During the early stages of the diagnostic process, doctors will request a blood test for creatine kinase (CK) levels. CK is an enzyme that leaks out when a muscle is severely damaged. When CK levels are elevated in a blood sample, it usually means that a muscle is destroyed by an abnormal process. Extremely high levels of CK indicate that the muscle themselves (not the nerves) are the cause of the weakness, but this does not identify the type of music disorder (“Muscular Dystrophy Association”, 2016).
Genetic Testing
Individuals can complete genetic testing to determine whether or not there is a mutation in the dystrophin gene. Females that are genetically related to males with DMD can undergo genetic testing to determine if they are carriers of the disease. It is extremely rare that females who are carriers will show symptoms of DMD (“Muscular Dystrophy Association”, 2016).
Muscle Biopsy A muscle biopsy is the surgical removal of a small sample of muscle to be examined by doctors for further understanding of muscle deterioration. Modern techniques can help distinguish muscular dystrophies from other disorders. For example, the amount of functional dystrophin protein found in a muscle biopsy can help determine whether the disease is likely to be DMD or Becker muscular dystrophy (“Muscular Dystrophy Association”, 2016).
Pathophysiology
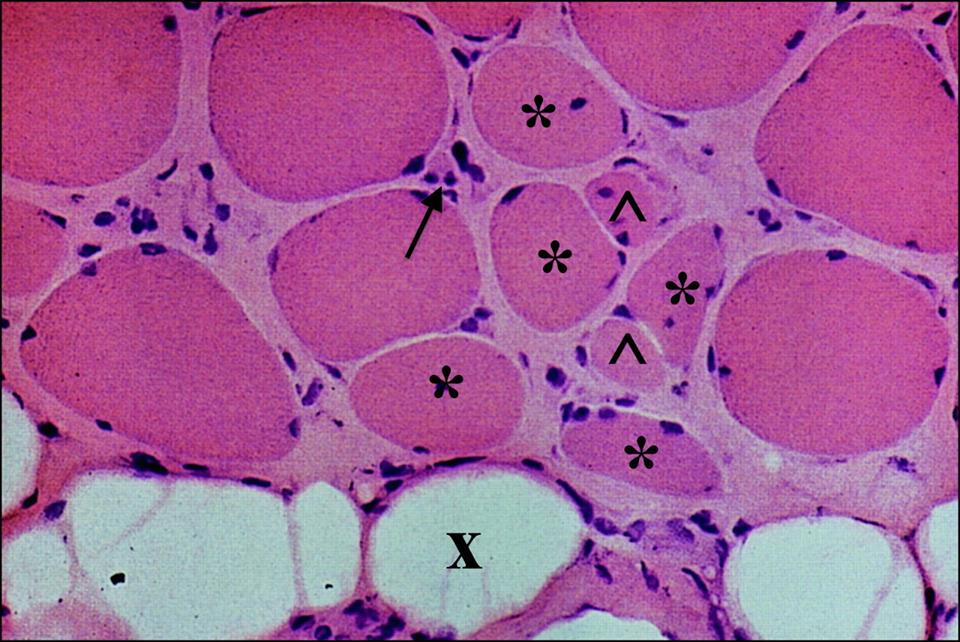
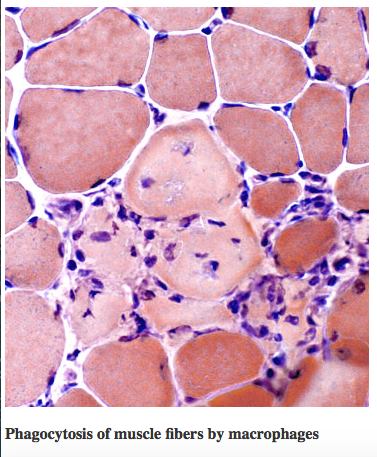
Muscle biopsies of patients diagnosed with DMD typically exhibit necrotic or degenerating muscle fibers, which often manifest in aggregate forms, and observable decreases in muscle fiber diameter (Deconinck & Dan, 2007). These necrotic fibers are surrounded by several macrophages and lymphocytes. During the early stages of the disease, small, immature muscle fibers are observed, which shows muscle regeneration from myoblasts, leading to a balance between necrosis and regeneration during these early phases. However, as the disorder progresses, the capacity of the muscle fibers to regenerate is greatly diminished; as a result, worn-out muscle fibers are gradually replaced with connective and adipose tissues. Thus, the symptomatical manifestations of DMD are due to the underlying imbalance between muscle fiber degeneration and myoblast regeneration, with the foremost pathologic feature being muscle tissue necrosis. In addition, studies on animal models of DMD suggest that the regenerative capability of the muscle fibers significantly decline with age of the individual (Deconinck & Dan, 2007).
Figure 2: Muscle biopsy of an individual with DMD. The * shows necrotic muscle fibers, which aggregates with other degenerating fibers, and shows a decreased muscle fiber diameter compared to healthy fibers. The arrow shows macrophages and lymphocytes that surround the necrotic fibers (or start to surround healthy muscle fibers). The ^ shows small, immature, fibers that arise from myoblasts during the early stages of the disease. The x shows connective and adipose tissue that replace worn-out fibers.
There are several hypothesized mechanisms that are proposed to lead to the muscle fiber necrosis seen in DMD (Deconinck & Dan, 2007):
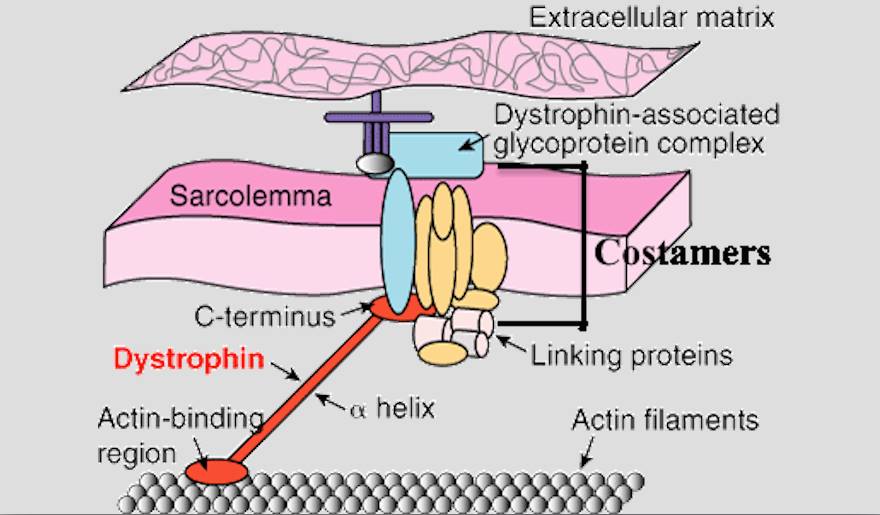
1.Mechanical Hypothesis. Dystrophin-associated protein complexes and additional proteins normally form lattice-like structures, called costamers, on the sarcolemmal cytoplasm. These costamers anchor the cytoskeleton components, such as the actin/myosin filaments, to the extracellular matrix, thereby maintaining the integral structure of the muscle fiber. In addition, costamers serve as mechanical couplers to distribute contractile forces generated in the sarcomere. The costamers are connected to the cytoskeleton components through the dystrophin protein. In individuals with DMD, mutations in the dystrophin gene lead to dysfunctional or insufficient copies (or lack thereof) of the dystrophin protein, which causes a delocalization of the dystrophin-associated proteins and thus alterations in the sarcolemmal membrane structure. This phenomenon underlies muscle fiber membrane fragility, as the cytoskeletal fragments lose their attachment to the membrane, leading to a disintegration of the integral framework and the internal contractile components of the muscle fiber. In addition, the ability to sustain eccentric muscular contractions appears to be drastically reduced in individuals with DMD (which serves as an indication of muscle weakness) compared to controls.
Figure 3 : Dystrophin, as well as Dystrophin-associated protein complexes (called Costamers), link cytoskeletal elements to the exoskeleton of the sarcolemma. In DMD, dystrophin is dysfunctional (or absent), leading to a disintegration of the internal framework of the muscle fiber.
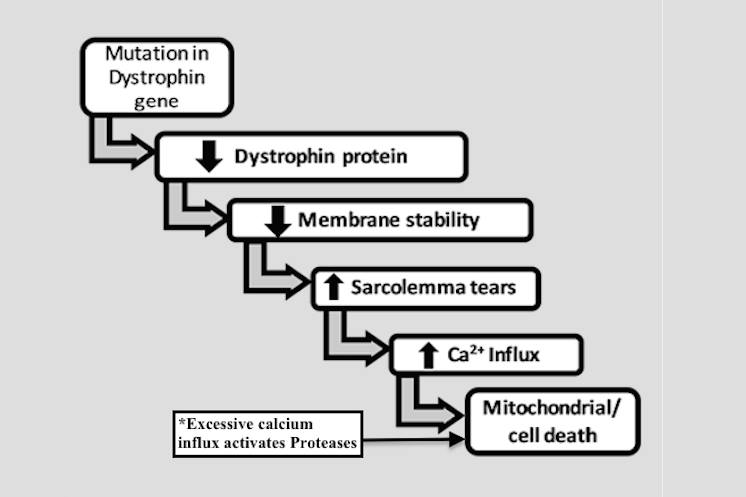
2. Calcium Hypothesis. Deficiencies in dystrophin protein in the sarcolemma lead to an increased calcium influx, mostly through the mechanosensitive calcium channels. Normally, this increased influx triggers calcium homeostatic mechanisms in order to maintain normal calcium concentrations. However, if excessive mechanical stress (i.e. loss of muscle fiber structure, as discussed above) occurs, which induces microlesions in the fiber membrane, high influx of extracellular calcium inevitably overrides the capacity of the muscle fiber to maintain physiologic calcium homeostasis. This leads to the activation of proteases, resulting in further disintegration of membrane components, ultimately leading to cell necrosis.
Figure 4: The calcium hypothesis of DMD
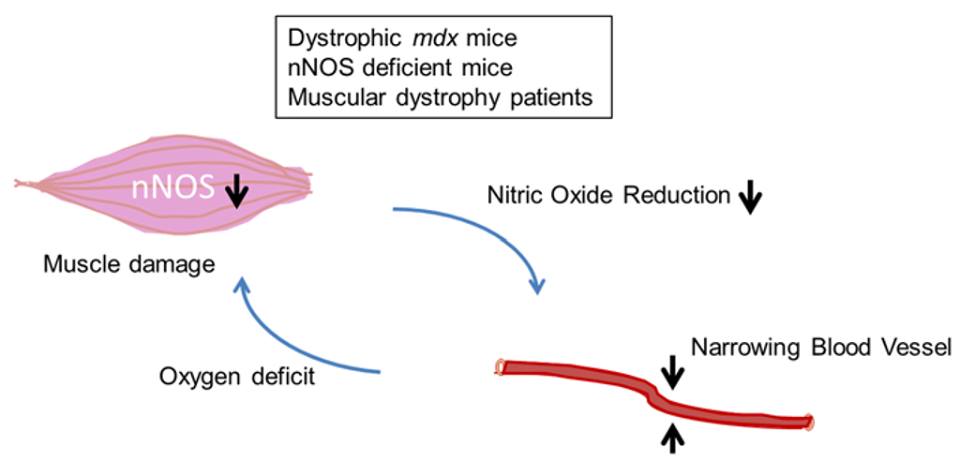
3.Vascular hypothesis. Earlier studies have proposed the possible role of abnormalities in muscular vasculature on the pathophysiology of DMD. However, structural studies have shown no such abnormalities. Rather, more recent insights into the local vasodilator role of nitric oxide and n-NOS in skeletal muscle may be more relevant to DMD. Nitric oxide is generated in skeletal muscle cells by the neuronal isoform of Nitric Oxide Synthase: n-NOS. In addition, n-NOS has an oxygenase domain, which plays a role in oxygen transport (i.e. oxidative phosphorylation). In muscles that have deficient dystrophin protein, n-NOS is delocalized from its subsarcolemmal anchorage, thus n-NOS floats freely in the cytoplasm, and its overall amounts in the sarcolemma is reduced. Therefore, during exercise conditions, when the need for oxygen transport in the sarcolemma is greatly increased, muscle ischemia may occur in DMD. The absence of sufficient n-NOS in the muscle fiber membrane also leads to reduced nitric oxide production, which causes vasoconstriction in the blood vessels, which further exacerbates the decrease in oxygen delivery to the muscle fibers. If prolonged oxygen deprivation is experienced, this eventually leads to increased susceptibility to metabolic stress, myofiber damage, and muscle fiber death.
Figure 5: The vascular hypothesis of DMD
4. Inflammatory Hypothesis. Muscle biopsies of patients with DMD consistently demonstrate inflammatory response upregulation. In these individuals, there have been an observed coordinated activity of numerous components of a chronic inflammatory response, such as cytokine and chemokine signaling, leukocyte adhesion and diapedesis, invasive cell type-specific markers, and complement system activation. In particular, CD4+ or CD8+ T cells or macrophages have been shown to aggravate the symptoms of the disease. However, the exact relationship between the immune response and disintegration of the muscle fiber components is yet to be investigated. One proposed mechanism is that these inflammatory agents phagocytize the muscle fibers, leading to muscle wasting. Also, most studies have provided little direct evidence on the mechanisms of inflammatory functions implicated in muscle fiber death.
Figure 6: The inflammatory hypothesis of DMD
Curent Treatments
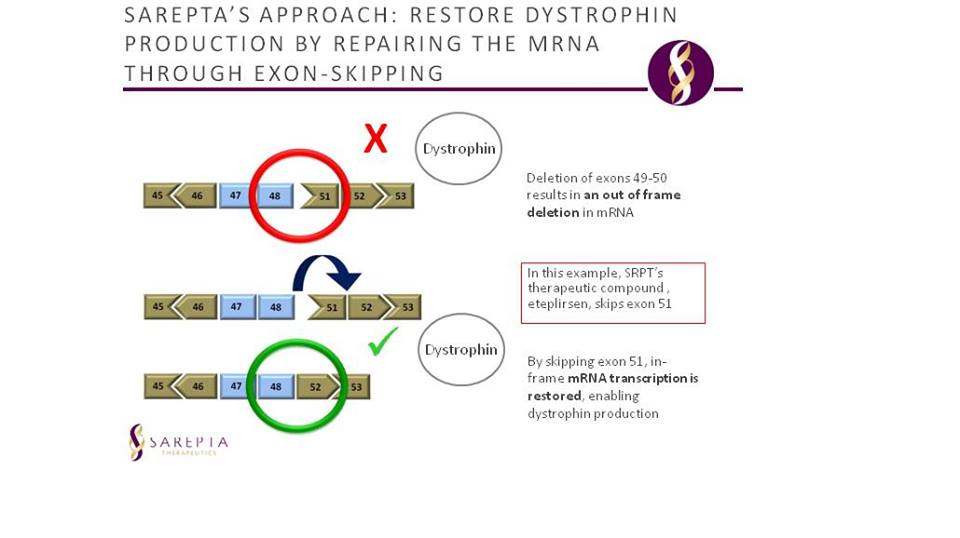
As there is no current cure for the disease, many of the present treatments aim to improve patient quality of life by slowing and controlling symptoms. However, this year, Eteplirsen (Exondys 51- Sarepta), an antisense oligonucleotide, given as an intravenous infusion, has received FDA accelerated approval for the treatment of DMD, specifically in patients who have an exon 51 dystrophin gene mutation. Eteplirsen causes exon 51 to be skipped downstream in the mRNA, which potentially aids in the production of a partially functional dystrophin protein by restoring the reading frame of the dystrophin mRNA. The accelerated approval of the drug is based on the trials showing promising results in study participants who showed an increase of dystrophin in skeletal muscle (FDA, 2016).
Figure 7: Eteplirsen mechanism shown; the exon skipping mechanism of the drug allows for a functional copy of dystrophin to be created.
Steriods Studies on patients using glucosteroids, such as prednisone, have shown treatment with the drug can increase muscle strength, ability, and respiratory function along with slowing muscle damage (American Thoracic Society, 2004). Deflazacort, an oxazoline derivative of prednisone, has been shown to have similar benefits to prednisone, but with possibly fewer side effects. Oral steroids are especially recommended for helping to preserve lung function, but more research is needed to confirm if and what exactly the potential pulmonary benefits of using the steroids are (American Thoracic Society, 2004).
Other Drugs
Additional drugs that patients take are for the protection and some prevention of cardiac muscle damage. Other drugs that seem to help are anticonvulsants to control muscle spasms, immunosuppressants to potentially delay some damage to dying muscle cells, and antibiotics to treat respiratory infections that occur frequently in patients.
Surgical Procedures
Some patients may need surgery to straighten the spine, repair shortened muscles or treat heart or lung specific issues. Specifically, a prophylactic surgery treatment aims to improve the likelihood of a wheelchair bound patient to potentially regain ability to stand or even walk with the help of a supporting appliance (Forst & Forst, 2012).
Maintenance
For patients to maintain an active and normal life as possible, it is highly recommended for patients to : - stand and walk as much as possible to keep bones strong and spine straight - maintain a healthy diet to prevent weight problems or help with constipation and swallowing issues; as any body functions relating to muscle movement are affected. - stay active to keep muscles and joints stretching and mobile In addition to these lifestyle recommendations, patients are encouraged to attend various therapies such as physical, respiratory, speech, and occupational therapy (NIH, 2016).
Future Treatments
Though there are different targets available for potential treatment of DMD, these treatments only manage the symptoms but do not stop the progression of the disease (Young et al., 2016). Currently researchers are investigating how stem cells could be used to treat DMD in the future. Another growing field of research involves gene editing. Therefore, scientists are looking at combining both methods to treat DMD in the future. Dystrophin is the largest human gene, which causes implications in gene therapy (Young et al., 2016). Additionally, cell therapy has limitations because skeletal muscle is the most abundant in the human body (Young et al., 2016). A potential gene editing treatment has been developed to treat 60% of DMD cases and is currently in clinical trials but further research is needed to ensure its efficacy and safety (Young et al., 2016). A technology called CRISPR/CAS9 is used to edit the genome by targeting specific gene sequences (Ledford, 2016). This method is able to detect a specific region of the dystrophin gene which creates 60% of the mutation in this gene (Young et al., 2016). This could potentially stop the progression of the disease and restore the lack of dystrophin protein (Young et al., 2016). These researchers obtained skin cells from DMD patients, and the cells were reprogrammed to create induced pluripotent stem cells (iPSCs) (Young et al., 2016). With the use of the gene editing technology, they were able to remove the mutations in the iPSCs and ultimately leads to dystrophin protein production (Young et al., 2016). These mutation-free iPSCs were then differentiated into either cardiac or skeletal muscle cells and were then implanted back into mice with a mutation in the dystrophin gene (Young et al., 2016). The results were that the transplanted muscle cells were able to successfully produce the dystrophin protein (Young et al., 2016). This was the first study to make the largest deletion in the dystrophin gene yet, so further studies are needed to test its efficacy and generalizability to humans (Young et al., 2016).
This must include at least 6 of the following symptoms of inattention that must have persisted for at least 6 months to a degree that is maladaptive and inconsistent with developmental level (American Psychiatric Association, 2013):
Single Photon emission computed tomography (SPECT)
SPECT is the most common testing technique to measure for ADHD. SPECT is a 20 minute neuroimaging technique that measures the blood flow within the brain. It shows regions in the brain that are active (hot) and quiescent (cold) while patients complete various tasks (Sherman, 2015).
Quantitative electroencephalography (qEEG)
qEEG measure brain activity by observing distinctive brain patterns. Patients wear an electrode-studded cap and gel is applied to the head to conduct electrical impulses (Sherman, 2015). The brain waves show physicians abnormalities in the frontal area of the brain. ADHD patients show excess slow waves while others may have way too fast-wave activity (Sherman, 2015). This testing helps physicians determine which medication to prescribe and which will be most effective.
Other
Other imaging tests that are currently being used to assist in the diagnosis of ADHD are positron emission tomography (PET) and magnetic resonance imaging (MRI). These brain scans reveal the significant sizes in the brain. However studies have shown that MRI scanning is not the best diagnostic tool to prove a patient has ADHD (Cai et al, 2015).
Symptoms
Many symptoms that occur can be seen in the DMS-5 criteria scale however other symptoms are appearance, mood, speech and thought process and cognition. Cognition being a common symptom includes having difficulty with calculation tasks and recent memory tasks. For adults, symptoms may be reckless driving, poor listening skills, marital problems, trouble relaxing and lateness.
Pathophysiology and Etiology
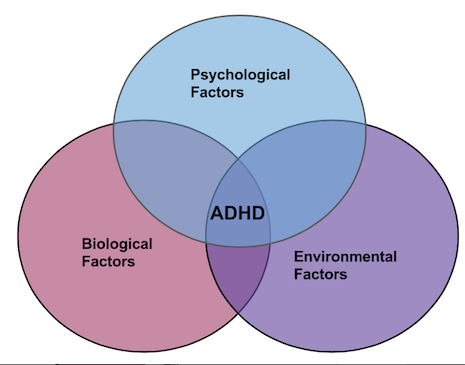
The biopsychosocial model of ADHD portrays a broad view which attributes the disorder outcomes to the interaction of three factors: biological, psychological, and environmental.
Pathophysiology of ADHD is unclear and the roots have yet to be discovered. Drugs used to treat ADHD have led to hypothesizing the involvement of certain areas in the brain involved in attention have some sort of neural transmission deficits. The underlying brain regions are thought to be involved distributed over various brain regions as opposed to a single area. Indicated though PET scan imaging, the most notable structural and functional alterations are in parietal lobes and cerebellum (Cherkasova & Hechtman, 2009). Specifically, the neurotransmitters dopamine and norepinephrine are associated with ADHD.
Psychological Factors
Temperamental Factors
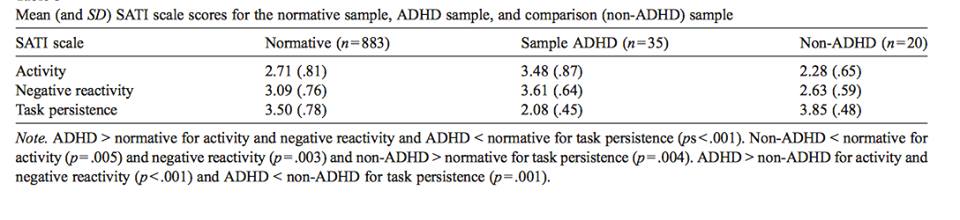
Temperament is the behavioral style that individuals consistently exhibit as a response to their environments (Foley, McClowry & Castellanos, 2008). It is evident early in life, relatively stable across time and various situations, biologically-driven and genetically-linked. For children with ADHD, three aspects of temperament are evident (Foley, McClowry & Castellanos, 2008): 1. Neuroticism (intensity and frequency of the expression of negative response to stress); 2. Conscientiousness (persistence in task performance); and 3. Extraversion/Activity (highly outgoing/overactive).
A study by Jensen and Rosen (2004) performed a study involving parent-ratings, where mothers of children with ADHD were asked to rate the intensity and frequency their child’s emotional responses. They found that children with ADHD were rated to be significantly more emotionally reactive to both immediate and future events compared to children without ADHD. In addition, children with ADHD showed a greater response to negative emotional events (both in immediate and future time periods) compared to positive emotional events. However, when rating the responses to the consequences of their behavior, children with ADHD were rated as less emotionally reactive than children without ADHD (Jensen & Rosen, 2004).
Hoza et al. (2001) investigated academic task persistence of boys with ADHD compared to boys without ADHD. The methodology involved a success-failure manipulation with find-a-word puzzles. Results show that boys with ADHD solved fewer test puzzles, had a higher frequency of quitting on the task, and found fewer words on a generalization task. In addition, boys with ADHD were observed to be less effortful and less cooperative in performing the task compared to the control group (Hoza et al., 2001).
Many children with ADHD (particularly the hyperactive type) are often described as ‘extreme extraverts’ or ‘over-active’ (Shaw, 2011). High levels of extraversion in ADHD has been linked to the insufficient amounts of dopamine in the brain regions linked to the reward-system. This chronic underarousal is hypothesized to be the underlying reason why these children subconsciously aim to compensate for this deficit by seeking more rewards through highly overt behavior. This excessive activity might also be due to their lack of motor inhibition; these children have trouble controlling movements due to deficiencies in gross and fine motor movements. Through measurements that track control of hand movements, children with ADHD appear to have tremendous difficulty in controlling unnecessary movements during motor tasks like simple finger-sequencing. As a result, these children show too much unnecessary movements, as well as difficulty in motor coordination and control, and have difficulty with performing a variety of tasks that involve motor skills, such as handwriting (Shaw, 2011).
Attention
There are three components of attention (Huang-Pollock, Nigg & Halperin, 2006): 1. *Selective attention* (the process of attenuating to only one thing at a time despite many non-relevant, distracting stimuli in the environment); 2. *Attentional orienting in space* (for instance, turning one’s attention towards a light stimulus flashing in one direction; and 3. *Arousal and vigilance* (the ‘readiness’ to pay attention in the event that a stimulus appears, or a state of ongoing arousal and alertness).
In individuals with ADHD, there are no apparent deficits in selective attention and attentional orienting in space. However, the arousal and vigilance components of attention are dysfunctional. In other words, these individuals have no impairments in arousal responses to stimuli, but rather they have deficits in their ‘preparedness’ in paying attention to these stimuli. When presented with a stimulus, they will not respond with the typical arousal responses as seen in control groups because of their low levels of alertness to pay attention to these stimuli (Huang-Pollock, Nigg & Halperin, 2006).
Response Inhibition
Response inhibition is the cognitive process of inhibiting oneself from making a dominant response when it is not appropriate in the situation (an example is a child with ADHD who persistently moves around the classroom despite being told by the teacher to sit quietly, and the rest of the children are sitting down). This process is impaired in individuals with ADHD. A study by Wodka et al. (2007) examined response inhibition in children with ADHD using three ‘go/no-go tests’: one with high cognitive demand, one with low cognitive demand, and one that is motivation-linked (have rewards and response costs). In ‘go/no-go’ tasks, test subjects are presented with a continuous stream of stimuli and they are asked to perform a binary decision on each stimulus. One of the stimuli requires participants to make a motor response (‘go’), whereas the other requires participants to withhold a response (‘no-go’). Accuracy and reaction times are measured for each event, and the dominant response is established by presenting a higher proportion of the ‘go’ stimuli, where the subjects are supposed to make the motor response (Wodka et al., 2007).
Results show that compared to control groups, children with ADHD made more errors in each of the simple, cognitive and motivation-linked go/no-go tests, i.e. over the course of several trials, these children made the ‘go’ response for the ‘no-go’ stimuli. These results show that the inability to inhibit the dominant response appears to be a primary deficit that is observed, even when the executive function demands of tasks are minimal, and even in the presence of rewards and motivational factors for performing the task correctly (Wodka et al., 2007).
Working Memory
Working memory is the component of short-term memory that is concerned with the immediate, conscious perceptual and linguistic processing (Barnett, Maruff & Vance, 2005). This process is impaired in ADHD, with huge deficits specifically in visuospatial working memory. A study by found that ADHD individuals demonstrated subtle but significant impairment in the performance of visuospatiial memory tasks. The memory impairment was delay-independent, which suggest dysfunction of the encoding rather than the retrieval phase of visuospatial memory. In other words, this may reflect attentional deficits rather than being due to the dysfunction of the explicit memory system (Barnett, Maruff & Vance, 2005).
Biological Factors
Family Studies
Increased rates of ADHD are often found in children of parents and siblings with ADHD.These correlating high risks indicates strong familial risk factors involved in the development of ADHD, whether that be genetic or environmental. Familial study findings have indicated additive genetic effects of ADHD within the range of 75-90%. This supports the understanding of some sort of underlying trait, most likely influenced by several rather than a single gene (Levy et al, 1997). In some family studies, it has also been found that low birth weight has a significant influence in twins (Freitag & Retz, 2011).
Adoption Studies
Adoption studies seek to identify the degree of influence on ADHD symptoms from environmental factors, although they can also be used to support the degree of influence of biological factors. This is done by comparing the prevalence of the disorder between the affected individual and their adoptive and biological relatives. If a disorder were to be environmentally influenced, then the individual should have a greater rate of correlation of symptoms with adoptive family. However, the findings of these studies can be limited due to many study designs not controlling for certain risk factors, such as the presence of maternal smoking in the adopted child’s biological parent (Freitag & Retz, 2011).
Twin Studies
Twin studies can provide insight into the extent of which a trait is due to genetic factors versus environmental and nurture factors. These studies are done by comparing the occurrence ADHD in monozygotic (MZ) twins, who share identical genomes, to dizygotic (DZ) twins, who share approximately half of their genes. Twin and family studies have shown a 60-80% concordance rate was determined (Freitag & Retz, 2010). Despite many twin studies having been done over the years with differing study designs, all results seem to be conclusively consistent. The nearly unanimous results have identified that individual differences in ADHD symptoms can be largely attributed to genetic influences, with an average heritability of .73 across all studies (Willcutt et al, 2010). However, most studies have been done on children with ADHD, identified as being between the ages of 8 and 18, and there is not much information currently on these twin studies having been done on adults (Freitag & Retz, 2011).
Candidate Gene Studies
Several quantitative genetic studies have suggested that genetic influences contribute significantly to the pathogenesis and development of ADHD (Gizer, Ficks & Waldman, 2009). Over the past 15 years, substantial efforts have been made in order to identify genes involved in the etiology of the disorder. Given that abnormalities in the dopamine neurotransmission has been suggested to underlie the etiology of ADHD, the genes that code for dopamine receptors have been identified as candidate loci for ADHD (Gizer, Ficks & Waldman, 2009). Two of the most studied genes are the the Dopamine D4 receptor gene (DRD4) and the Dopamine transporter gene (DAT1).
DRD4 gene
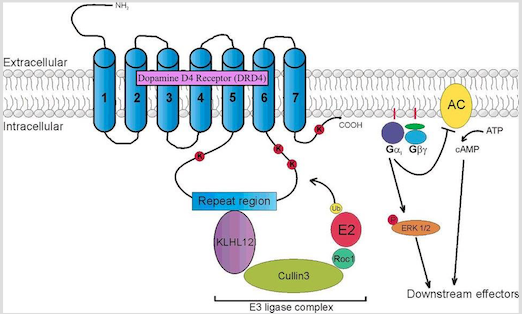
DRD4 is a G protein-coupled receptor belonging to the dopamine D2-like receptor family, which act to inhibit adenylyl cyclase (Gizer, Ficks & Waldman, 2009). The DRD4 gene has been mapped to position 15.5 of the p-arm of chromosome 11. It is mostly expressed in the frontal lobe regions of the brain, such as the orbitofrontal cortex and anterior cingulate, thus playing a role in the regulation of higher-level cognitive functions such as reward anticipation, decision-making, impulse control, and emotion. Association studies have linked this gene to the personality trait of novelty seeking, and has been likened to the high levels of impulsivity and excitability seen in the diagnosis of ADHD. In addition, DRD4 knockout mice have been shown to exhibit a heightened response to cocaine and metamphetamine compared to control groups, as seen through increases in their locomotor activity. The most widely studied DRD4 polymorphism is the 48-bp variable number of tandem repeats (VNTR) in exon 3 of the gene. This VNTR has been shown to play a role in secondary messenger systems (i.e. cyclic AMP) and in responses to antipsychotic medication such as clozapine (Gizer, Ficks & Waldman, 2009).
DAT1 gene
Environmental Factors
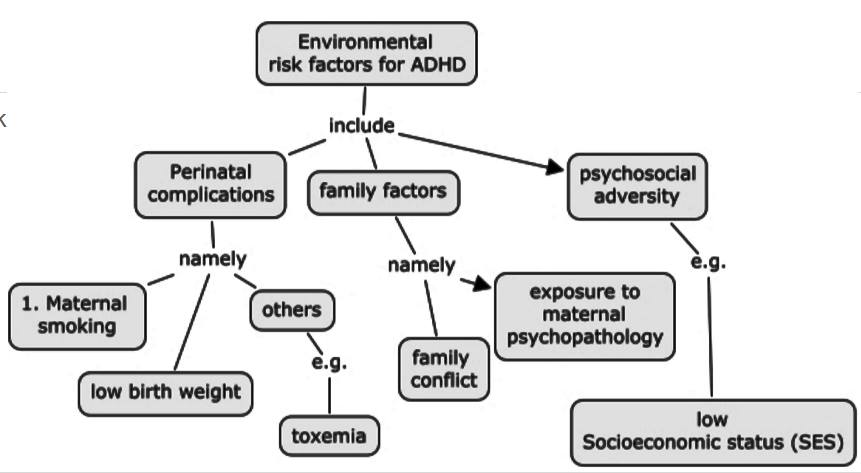
Various environmental factors are thought to contribute as risk factors to the development of ADHD such as including food additives/diet, pre- and postnatal toxin exposure or mercury or lead, cigarette and alcohol exposure, maternal smoking during pregnancy, and low birth weight. Many recent studies have specifically examined the relationships between ADHD and these extraneous factors (Banerjee et al, 2007). However, it is to be noted that these studies show that these environmental risk factors and potential gene–environment interactions increase the risk for developing ADHD. In addition to this, even if one does not have a high expression of the candidate genes of risk, but were exposed to an environmental risk factor, there is also a risk for developing ADHD.
Treatment
Methylphenidate (e.g. Ritalin)
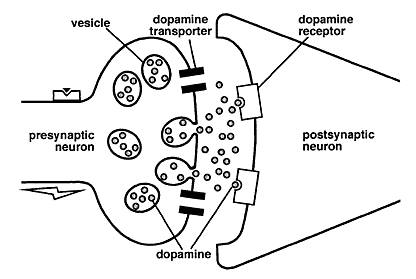
Methylphenidate (MPH) is the most commonly prescribed drug for the treatment of ADHD (Volkow et al, 2002). It is a stimulant drug that blocks dopamine and norepinephrine transporters in the brain, thereby increasing the levels of dopamine in the brain (Volkow et al, 2002).
Though the exact mechanism of action is widely unknown, it may help increase attention and decrease impulsiveness and hyperactivity (FDA, 2013). Methylphenidate potentiates, or strengthens, dopaminergic signalling by increasing the effect of dopamine cell firing on the concentration of dopamine in the synaptic cleft (Tripp & Wickens, 2008).
The Dopamine Transfer Deficit (DTD) theory suggests that there are specific alterations in the magnitude and timing of this anticipatory dopamine cell firing in children with ADHD (Tripp & Wickens, 2008). This anticipatory dopaminergic cell firing is important for behavioural reinforcement in learning (Tripp & Wickens, 2008). The DTD theory predicts that for children with ADHD, there is a dopamine transfer dysfunction. Specifically, the phasic dopamine cell response to the cue that predicts reinforcement is reduced in amplitude to the point of being ineffective (Tripp & Wickens, 2008).
Consistent with the DTD theory, it is beneficial to block the dopamine transporter, DAT, so there is less dopamine reuptake and more dopamine present in the synaptic cleft (Tripp & Wickens, 2008). This type of drug treatment should not be used for children under six years of age because it has not yet been studied for this age group (FDA, 2013). Some side effects of Ritalin include, headache, decreased appetite, stomach ache, nervousness, trouble sleeping and nausea.
Dexmethylphenidate
Most cases of ADHD persist into adulthood. This dug is a safe and effective treatment for ADHD among children and adults (Spencer et al, 2007).
Its mechanism of action is more known and is similar to methylphenidate. This drug binds to and blocks the presynaptic dopamine transporter in the basal ganglia, resulting in an increased amount of dopamine in the synaptic cleft (Spencer et al., 2007).
In preclinical studies, the drug raised extracellular levels of dopamine (Spencer et al., 2007). This form of treatment is available in tablet form and should be taken 2-3 times daily due to the short-half life (Spencer et al., 2007).
It was noted that dopamine levels were elevated in the nucleus accumbens as well (Bymaster et al., 2002). The nucleus accumbens is involved in the reward circuit, thereby increasing the susceptibility of abuse of psychostimulants; stimulants are considered a Schedule II drug meaning that there is a high potential for abuse (Allen et al., 2005).
The downside is that some children reported to feel embarrassed at school when taking the medication and would often skip it (Tripp & Wickens, 2008).
Another pitfall is that individuals with ADHD are often forgetful and may miss a dosage of their medication and thus impairs the efficacy of the drug (Tripp & Wickens, 2008).
In a specific study, the only side effects experienced were a dry mouth (Spencer et al., 2007).
Adderall
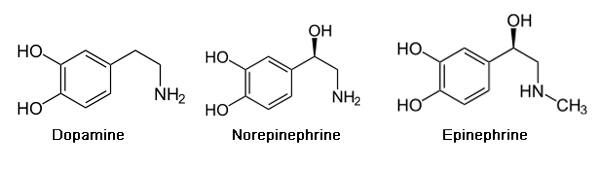
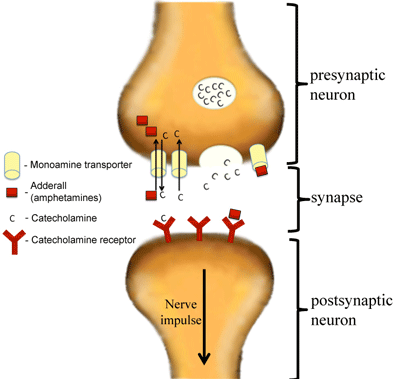
Adderall is a chemical compound that is made up of a mixture of the amphetamine salts that is used to enhance attention (Sherzada, 2012). The function of Adderall is to block the reuptake of norepinephrine, dopamine, and serotonin into the presynaptic neuron and increase the release of these monoamines into the synaptic space (Sherdzada, 2012). Monoamines include catecholamines (epinephrine, norepinephrine and dopamine). Dopaminergic activity is enhanced, again by binding to DAT, the dopamine transporter, and inhibiting the reuptake of dopamine to increase levels of dopamine in the synaptic cleft (Sherzada, 2012).
Normally, neurotransmitters such as dopamine are stored in vesicles in the presynaptic neuron, and are released when triggered by an action potential. Amphetamine enters the presynaptic neuron and forces dopamine to be released from the vesicles and into the synaptic cleft (Sherzada, 2012). Essentially, the dopamine transporter DAT is working in reverse, so as to release dopamine rather than reuptake it.
This reduces the symptoms of inattention, impulsivity and hyperactivity. Some possible side effects in children and adolescents include loss of appetite, insomnia, abdominal pain, nausea and vomiting (Sherzada, 2012). Adults may experience dry mouth, loss of appetite, insomnia, headache, weight loss, nausea, anxiety, agitation, dizziness, tachycardia, diarrhea, asthenia, and urinary tract infections (Sherzada, 2012).
References
American Thoracic Society. (2004). Respiratory Care of the Patient with Duchenne Muscular Dystrophy. American Journal of Respiratory and Critical Care Medicine, 170(4), 456–465. https://doi.org/10.1164/rccm.200307-885ST
Cordier, L., Hack, A. A., Scott, M. O., Barton-Davis, E. R., Gao, G. P., Wilson, J. M., … & Sweeney, H. L. (2000). Rescue of Skeletal Muscles of [gamma]-Sarcoglycan-Deficient Mice with Adeno-Associated Virus-Mediated Gene Transfer. Molecular Therapy, 1(2), 119.
DA. (2016). Press Announcements - FDA grants accelerated approval to first drug for Duchenne muscular dystrophy [WebContent]. Retrieved November 22, 2016, from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm521263.htm
Deconinck, N., & Dan, B. (2007). Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatric neurology, 36(1), 1-7.)
Duchenne Muscular Dystrophy (DMD) - Muscular Dystrophy Association. (2016). Muscular Dystrophy Association. Retrieved 2 November 2016, from https://www.mda.org/disease/duchenne-muscular-dystrophy
Forst, J., & Forst, R. (2012). Surgical treatment of Duchenne muscular dystrophy patients in Germany: the present situation. Acta Myologica, 31(1), 21–23.
Human Diseases and Conditions. (2016).Muscular Dystrophy. Retrieved November 23, 2016, from http://www.humanillnesses.com/original/Men-Os/Muscular-Dystrophy.html
Ledford, H. (2016). CRISPR: gene editing is just the beginning. Nature, 531(7593), 156-159. http://dx.doi.org/10.1038/531156a
New York Stem Cell Foundation. (2015). New York Stem Cell Foundation Invents Robotic Platform for Making Induced Pluripotent Stem Cells. Beyond the Dish. Retrieved 21 November 2016, from https://beyondthedish.wordpress.com/2015/08/08/new-york-stem-cell-foundation-invents-robotic-platform-for-making-induced-pluripotent-stem-cells/
Muscular Dystrophy Association. (2016). Duchenne Muscular Dystrophy (DMD). Retrieved November 23, 2016, from https://www.mda.org/disease/duchenne-muscular-dystrophy
NIH. (2016). Muscular Dystrophy: Hope Through Research. Retrieved November 23, 2016, from http://www.ninds.nih.gov/disorders/md/detail_md.htm
NIH. (n.d.). What are the treatments for muscular dystrophy? (n.d.). Retrieved November 21, 2016, from https://www.nichd.nih.gov/health/topics/musculardys/conditioninfo/pages/treatment.aspx
Thompson, J. D. (2016). Muscular Dystrophy. Retrieved November 23, 2016, from http://emedicine.medscape.com/article/1259041-overview
Young, C., Hicks, M., Ermolova, N., Nakano, H., Jan, M., & Younesi, S. et al. (2016). A Single CRISPR-Cas9 Deletion Strategy that Targets the Majority of DMD Patients Restores Dystrophin Function in hiPSC-Derived Muscle Cells. Retrieved 18 November 2016, from http://www.nature.com/news/crispr-gene-editing-is-just-the-beginning-1.19510