This is an old revision of the document!
Table of Contents
Alzheimer's Disease
Introduction
Alzheimer’s disease (AD) is an irreversible brain disorder that is characterized by a progressive decline in cognitive function, which typically begins with deterioration in memory (Alzheimer’s Disease, 2016). Alzheimer’s is the most common neurodegenerative disorder, and is the sixth leading cause of death in the United States (Alzheimer’s Disease, 2016). The greatest known risk factor is increasing age, however Alzheimer’s is not a normal part of aging as one can also develop early-onset Alzheimer’s in their 40s or 50s of age (Alzheimer’s Disease, 2016). Alzheimer’s disease is a degeneration of neurons in the brain, starting in the temporal lobe and spreads to parietal, and frontal lobe(Alzheimer’s Disease, 2016)). The overall mass of the brain is reduced as a result of cell death, and the degeneration of neurons (Alzheimer’s Disease, 2016)). Alzheimer’s disease is progressive, and has three stages : early, mild to moderate, and severe (Alzheimer’s Disease, 2016)). In the earlier stages, memory loss is mild compared to the late stages, where the ability to carry a conversation and respond to the environment is completely impaired, resulting in complete dependence on others for care (Alzheimer’s Disease, 2016). There is no cure for Alzheimer’s that stops the progression completely, however treatments are available for the symptoms and can temporarily slow the worsening of the symptoms, and improve quality of life (Alzheimer’s Disease, 2016).
History
The history of Alzheimer’s disease dates back to 1906 (Hippius & Neundörfer, 2003). Alois Alzheimer’s was a German psychiatrist and neuropathologist. In 1901, Alzheimer’s observed a female patient, Auguste Deter who was 51-year-old, and had unpredictable behavioural symptoms including a loss of short-term memory (Hippius & Neundörfer, 2003). Deter became Alzheimer’s obsession, and he requested that she remained at the Frankfurt asylum where he was able to receive her records and brain upon her death in 1906 (Hippius & Neundörfer, 2003). Alzheimer’s used staining techniques to examine the brain, and identified amyloid plaques and neurofibrillary tangles present (Hippius & Neundörfer, 2003). These brain abnormalities identified were concluded to be indicators of Alzheimer’s disease as a result of Dr. Alois Alzheimer’s obsession (Hippius & Neundörfer, 2003). The diagnosis of Alzheimer’s disease was initially specific for individuals between the ages of 45 and 65 who developed symptoms of dementia. The terminology changed after 1977, which had led to the diagnosis of Alzheimer’s disease independent of age (Hippius & Neundörfer, 2003). Senile dementia of the Alzheimer type (SDAT) was a term used to describe the condition for individuals over the age of 65, and classical Alzheimer’s disease used to describe those that were younger (Hippius & Neundörfer, 2003). Eventually, the term Alzheimer’s disease was adopted to describe individuals of all ages with a characteristic common symptom pattern, disease course, and neuropathology (Hippius & Neundörfer, 2003).
Epidemiology
Globally, the prevalence of Alzheimer’s disease is estimated to be 24 million, and is predicted to double every 20 years (Mayeux & Stern, 2012). North America’s population is aging, and as a result of this, there is an exponential rise of patients diagnosed with Alzheimer’s disease as seen in Figure 1 (Mayeux & Stern, 2012). The highest prevalence of Alzheimer’s is in North America, Western Europe, Latin America, and China (Reitz, Brayne & Mayeux, 2011). The average age when patients are affected is at 60 years (Reitz et al., 2011). The annual incident rates (per 1000) in these countries are 10.5 in North America, 8.8 in Western Europe, 9.2 in Latin America, and 8.0 in China (Reitz et al., 2011).
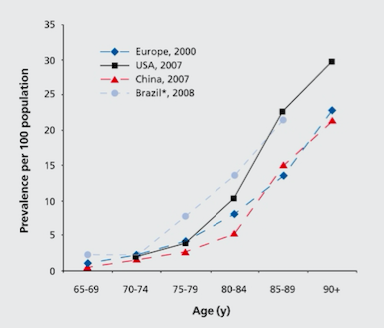 Figure 1: The graph depicts the prevalence of AD across Europe, China, USA, and Brazil in the elderly population.
Figure 1: The graph depicts the prevalence of AD across Europe, China, USA, and Brazil in the elderly population.
Studies have shown that although advancing age is a risk factor, there is also a sex difference in incidence and prevalence rates (Bermejo-Pareja, Benito-León, Vega, Medrona, & Román, 2008). Research shows that women have a higher risk of developing AD in the population older than 85 years of age (Figure 2) (Bermejo-Pareja et al., 2008). Incidence figures increase with age in women, but decreased beyond age 90 in men.
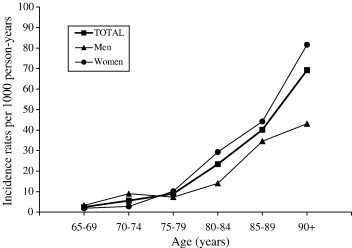 Figure 2: The graph compares the incidence rates of AD in men and women in the same age groups.
Figure 2: The graph compares the incidence rates of AD in men and women in the same age groups.
Etiology
Several risk factors have been suggested as causative agents for Alzheimer’s that are associated with the etiology and outcome of the disease such as:
- Cerebrovascular Disease - Changes such as hemorrhagic infarcts, small and large ischemic cortical infarcts, and white matter changes all increase the risk of dementia. Stroke can lead to cognitive impairment and Alzheimer’s as it can directly damage brain regions important in memory function such as thalamus. Stroke can also increase the amyloid-β deposition, which can lead to cognitive decline (Reitz et al., 2011)
- Hypertension - Increase risk of AD by decreasing the vascular integrity of the blood-brain barrier, which results in protein extravasation into brain tissue. This can lead to cell damage, reduction in neuronal or synaptic function, apoptosis, and an increase in amyloid-β deposition, resulting in cognitive impairment (Reitz et al., 2011)
- Smoking- Increases generation of free radicals, leading to high oxidative stress, which leads to activation of phagocytes and further oxidative damage. Smoking can also promote cerebrovascular disease, which is a risk factor for AD (Reitz et al., 2011)
Other risk factors include:
- Family History - Those who have a family member that has AD is more likely to develop the disease. Risk increases if more than one family member has the illness (Reitz et al., 2011).
- Genetics (Hereditary):
- Risk Genes - Increase likelihood of developing a disease. Research shows several genes that increase of AD such as the apolipoprotein E-e4 (APOE-e4) and this has the strong impact. If one copy of APOE-e4 is inherited, there is an increased risk of developing AD, and inheriting two copies results in an even higher risk (Reitz et al., 2011.
- Deterministic Genes- Directly causes a disease. Variations of the genes coding three proteins directly cause AD, and this includes amyloid precursor protein (APP), presenilin-1 (PS-1), and presenilin-2 (PS-2). When AD is caused by a deterministic variation, it is known as familial AD (Reitz et al., 2011).
Diagnosis
Fluro-deoxy-D-Glucose Positron Emission Tomography (FDG PET):
Flurodeoxyglucose is a glucose analog which is labeled with Fluorine-18 (half-life 110 min), and takes advantage of the brain’s primary fuel source in order to capture synaptic activity via PET scan (Johnson et al., 2012). Normal cerebral glucose metabolism has been established with aging, making changes that occur in early AD and throughout, precise through this type of PET scan (Marcus et al., 2014). FDG is a sufficient biomarker for overall brain metabolism as seen with evidence in a study by Rocher et al. in 2003, which illustrated that FDG uptake strongly correlated with levels of synaptic vesicle protein synaptophysin, found during autopsy. Over the years, many studies have proved the efficacy of recognizing FDG-PET scans to better aid with the diagnosis of Alzheimer’s. Along with a recognizable endophenotype for AD, there are resembling hypometabolic regions of the brain that are characteristic in established AD patients (Johnson et al., 2012). The metabolic deficiencies can be seen in Figure 3, showing various regions of the parietotemporal association cortices, posterior cingulate cortex, and the precuneus, reduced due to decreased synaptic activity, thus resembling the FDG-PET endophenotype (Johnson et al., 2012; Marcus et al., 2014). A meta analysis by Bloudek et al., 2011, showed that out of 119 modalities used to diagnose AD, FDG-PET had superior diagnostic capability as compared to MRI, CT, SPECT and biomarkers.
 Figure 3: A FDG-PET scan outlining the endophenotype typical of an Alzheimer’s patient in the mild to moderate stage of AD. The precuneus, lateral parietal, lateral temporal, and medial temporal lobes have slowly decreased in neuronal mass over time thus displaying decreased glucose metabolism.
Figure 3: A FDG-PET scan outlining the endophenotype typical of an Alzheimer’s patient in the mild to moderate stage of AD. The precuneus, lateral parietal, lateral temporal, and medial temporal lobes have slowly decreased in neuronal mass over time thus displaying decreased glucose metabolism.
Signs, Stages & Symptoms
Alzheimer’s disease is progressive and composed of different stages, characterized by different symptoms that also worsen over time (Alzheimer’s Disease, 2016).
Pre-Clinical AD: Early Stage (7-20 years)
Changes may start to happen as long as twenty years or more before a clinical diagnosis, such as the slow formation of plaques and tangles causing short-term memory loss (Alzheimer’s Association, n.d). Also known as the pre-dementia stage or mild-cognitive impairment (MCI), patients may experience a hinderance in both learning new information and their ability to access the semantic memory system (Förstl & Kurz, 1999). As such, the patient may slowly forget familiar words, colours, material they just read, misplace valuable objects and have trouble planning and organizing (Alzheimer’s Association, n.d). Also at this stage, clinically, there is no differentiation between the early stages of Alzheimer’s Disease (AD) and other non-AD memory impairments that may be benign and/or reversible, causing unreliability in detecting and providing treatment as early as possible (Arlt, 2013). The brain areas affected in this stage can be seen in Figure 3 and are the entorhinal cortex and the hippocampus, in charge of thinking/planning, and learning/memory respectively. Some alternate distinctive changes that may be seen are short term memory loss and the shrinkage of the amygdala (Alzheimer’s Association, n.d).
Early Stage Symptoms:
- Short-term memory loss
- Language problems characterised by a decreased vocabulary and word fluency but patient with AD can communicate basic ideas adequately
Mild to Moderate AD: Mid-Stage (2-4 years)
Also known as the mild to moderate dementia stage, most AD patients are diagnosed in this stage. There is an observed increase in plaques and tangles found in the hippocampus and frontal lobe, causing memory hinderance serious enough to interfere with work or social life (Alzheimer’s Association, n.d). Moreover, plaques and tangles will spread to the speaking/understanding speech as well as the spatial orientation areas of the brain, resulting in the shrinkage of the cortical area, shrinkage of hippocampus and moderately enlarged ventricles (Alzheimer’s Association, n.d). In the early parts of this stage, patients may be able to live independently, however as the disease progresses, patients tend to “live in the past”, language, reading and writing skills worsen and patients gradually lose insight into their own condition— so close supervision is necessary (Förstl & Kurz, 1999). As Alzheimer's progresses, individuals may experience changes in personality and behaviour as well, resulting in trouble recognizing family members (Alzheimer’s Association, n.d). Due to this, family support seems to diminish due to restlessness, aggression, disorientation and involuntary urinary excretion (Förstl & Kurz, 1999). At the later times of this stage, closer to the Severe Stage, the parietal, frontal and temporal lobes will all be affected with this disease.
Mid-Stage Symptoms:
- Memory loss continues
- Confusion of location of familiar place
- Taking longer to accomplish normal daily tasks
- Poor judgement
- Language impairment
- Mood changes
Severe AD: Late Stage (~3 years)
In this stage, almost all cognitive functions are severely impaired, while the brain has decreased in mass significantly due to widespread cell death (Alzheimer’s Association, n.d). Moreover, the extreme shrinkage particularly in the cerebral cortex and hippocampus is accompanied by a large increase in the ventricles of the brain (Alzheimer’s Association, n.d). The language of patients is severely reduced to simple phrases, however emotions can still be broadcasted or received (Förstl & Kurz, 1999). Nursing support is also disrupted due to many reasons. One being the patient's inability to understand nursing interventions, leading to angered experiences, along with displays of apathy and exhaustion (Förstl & Kurz, 1999). Also, due to impaired motor functions (chewing and swallowing) and large disturbances, nurses may have a harder time feeding AD patients (Förstl & Kurz, 1999). Moreover, plaques and tangles are widespread throughout the brain now, and severe dementia, and weight loss due to bed-riddance are often observed in the Severe AD stage (Alzheimer’s Association, n.d).
Late Stage Symptoms:
- Memory is completely lost
- Language is jumbled
- Autonomic functions are compromised
- Loss of mobility
- Weight loss
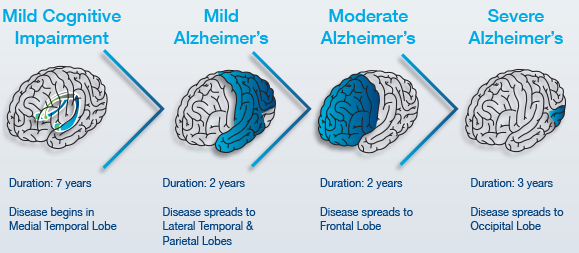 Figure 3: The stage by stage progression of Alzheimer's and its spatial and temporal infection patterns in the brain.
Figure 3: The stage by stage progression of Alzheimer's and its spatial and temporal infection patterns in the brain.
Pathophysiology
Alzheimer's disease is characterized by neuronal loss, decrease in cortical neurons and synapses in the brain. This results in the degeneration of an individual's cognitive capacity. From numerous studies, it was identified that amyloid plaques and Tau proteins are heavily concentrated in AD patient's brains. Thus, implying a significant involvement in the progression of AD (Ballatore et al., 2007).
Tau Proteins
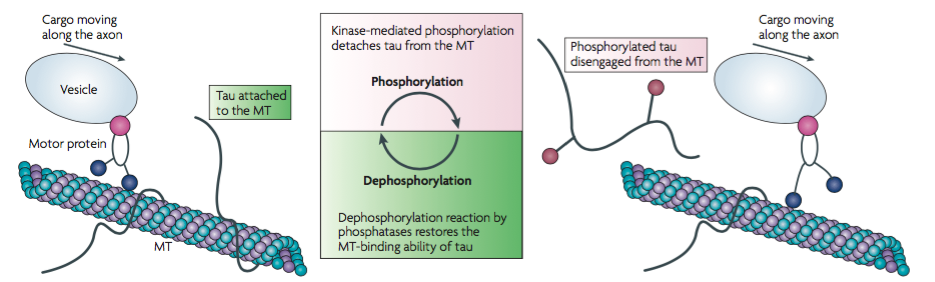 Tau proteins are microtubule-associated proteins (MAPs) found on the surface of microtubules in a neuronal cell. They stabilize the microtubules and prevent depolymerization to allow for the transportation of essential nutrients across the axon (Delacourte & Defossez, 1986). Tau proteins allow for normal axonal growth and maintenance of the internal structure of the neuronal transport system. Protein kinases such as GSK3 and CDK5 are involved in the phosphorylation of Tau proteins. The kinases cause Tau proteins to detach from the microtubules to allow cargo to move across the axon. After the cargo has safely transported across the axon, a dephosphorylation reaction occurs by which phosphatases reattach Tau proteins to the microtubules (Ballatore et al., 2007).
Tau proteins are microtubule-associated proteins (MAPs) found on the surface of microtubules in a neuronal cell. They stabilize the microtubules and prevent depolymerization to allow for the transportation of essential nutrients across the axon (Delacourte & Defossez, 1986). Tau proteins allow for normal axonal growth and maintenance of the internal structure of the neuronal transport system. Protein kinases such as GSK3 and CDK5 are involved in the phosphorylation of Tau proteins. The kinases cause Tau proteins to detach from the microtubules to allow cargo to move across the axon. After the cargo has safely transported across the axon, a dephosphorylation reaction occurs by which phosphatases reattach Tau proteins to the microtubules (Ballatore et al., 2007).
Hyperphosphorylation of Tau proteins results in the formation of neurofibrillary tangles (NFTs). When Tau proteins are hyperphosphorylated, they dissociate from microtubules, thus destabilizing them (Ballatore et al., 2007). The structure of the microtubules deteriorates such that they can no longer actively participate in the neuronal transport system. Free roaming Tau proteins aggregate to form NFTs which usually begin to form at the temporal lobe of the brain. Tau proteins can no longer be dephosphorylated to reattach to the degraded microtubules and the neuronal cell is ultimately destroyed (Ballatore et al., 2007). Research shows that the location and density of NFTs play a critical role in the pathophysiology and progression of AD. The direction of progression can vary and thus initial symptoms of AD vary between individuals (Ballatore et al., 2007).
Therapeutics for Alzheimer’s Disease
The copious complex mechanisms involved in the pathogenesis and pathophysiology of Alzheimer’s Disease (AD) creates a challenge to innovate effective therapeutics to delay or halt the disease progression (Fig.1) (Anand, 2014). However, extensive scientific efforts have been devoted into pharmacotherapeutic research for the treatment of AD. The development of present therapeutics have been based upon the first theory proposed to explain the disease known as the cholinergic hypothesis (Francis, 1999). This study evidently reveals the selective loss of cholinergic neurons in the nucleus basalis, which results in reduction in cholinergic activity among patients with AD. (Whitehouse et al., 1981). Additional studies confirm that AD may be associated with diminution of cholinergic activity, further studies utilized rhesus monkeys to show the effects of anticholinergic scopolamine on memory deficits as seen in AD (Bartus, 1978). Therefore, the development of therapeutics to augment cholinergic activity such as cholinesterase inhibitors (CIs) have primarily been focused upon. The CI’s function to enhance the cholinergic transmission by reducing in the breakdown of acetylcholine (ACh). For this reason, elevated levels of ACh are presented between neuronal synaptic clefts, thereby, compensating for the attenuated levels of ACh due to cholinergic neuronal death (Stahl, 2000). Currently, four Cis for symptomatic treatment of the disease have been approved by the FDA – donepezil, rivastigmine, galantamine, tacrine (Lleo, 2007). Such therapies have been recognized as first-line of treatment for mild to moderate progression of AD, though, tacrine is no longer used in clinic due to its associated effects of tacrine-induced liver damage (Alfirevic, 2007). A systematic review and metanalysis conducted by Hansen et al. has shown that donepezil, galantamine, and rivastigmine have shown overall benefits for stabilizing or slowing decline in cognition, function, behavior and clinical global change (Hansen, 2008). Despite the benefits of CI for moderate AD, the elevation in the levels of ACh can result in cholinergic adverse effects among patients which include nausea, vomiting, diarrhea, bradycardia, muscle cramps, and insomnia (Ellis, 2005). Moreover, memantine – an N-methyl D-aspartate (NMDA) receptor antagonist provides a treatment option for moderate to severe cases of AD (Yiannopoulou, 2013). Such drug serves to preclude the excessive release of an excitatory neurotransmitter glutamate resulting in excitotoxicity (Aprahamian, 2013). Moreover, a study by McShane et al. shows the benefit of memantine in moderate to severe to severe AD, which includes improvement on cognition, activities of daily living, and behavior at six months (McShane, 2006). However, although memantine is shown to be well tolerated in clinical studies, it can commonly be associated with side effects such as dizziness, constipation, confusion, headaches, hypertension, comnolence and visual hallucinations (McShane, 2006; Gauthier, 2006 ). While CI’s have been used as a standard mode of treatment to offer palliative care, ongoing research has been conducted for treatments capable of halting or modifying the progression of AD (Yiannopoulou, 2013).
Future Research & Implications
Targeting Tau Aggregates
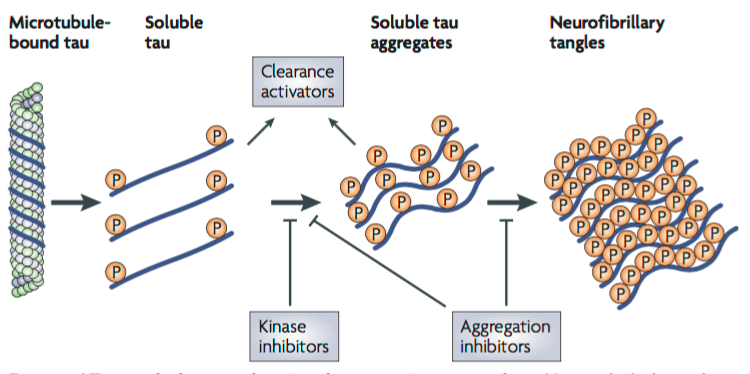
Tau is a soluble microtubule-binding protein. One of the functions of Tau is to stabilize microtubules in axons for axonal transport, and as cytoskeletal elements for growth (Citron, 2010). One of the characteristics observed in AD neurons consist of hyperphosphorylated, aggregated insoluble tau (Citron, 2010). This leads to direct toxic effects such as a loss of axonal transport as tau can be detached from microtubules leading to the formation of soluble tau aggregates forming neurofibrillary tangles (Citron, 2010). Current therapeutic strategies focus on the inhibition of tau aggregation, and to block tau hyperphosphorylation (Citron, 2010). One of these strategies is to design kinase inhibitors, which would prevent hyperphosphorylation, and design aggregation inhibitors that would block the soluble tau aggregates and formation of tangles (Citron, 2010). Tau toxicity can also be prevented by enhancing clearance of tau, and degradation of tau aggregates (Citron, 2010).
Targeting Aβ Plaques
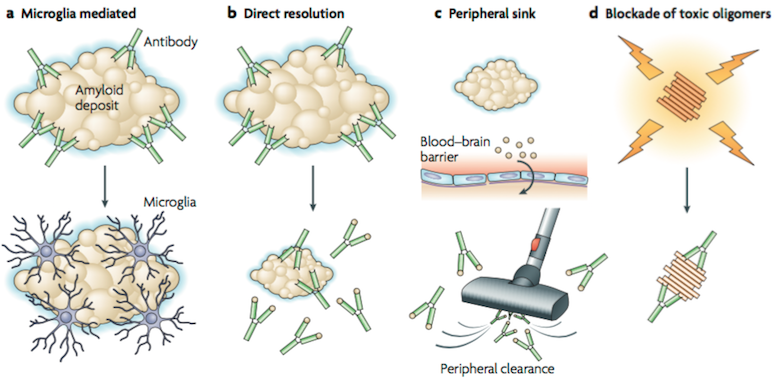
Over the past few years, amyloid-β (Aβ) immunotherapy have become a fascinating area of research in AD. Research in this field was initiated after the publication of the first immunization paper from Elan that reported that amyloid pathology was reduced in an APP transgenic mouse model after vaccination with aggregated Aβ (Citron, 2010). Three hypotheses have been proposed regarding Aβ immunotherapy mechanism. The first mechanism (Figure A) is based on microglial activation and phagocytosis. In this mechanism, amyloid-specific antibodies are administered and reach the central nervous system, bind to amyloid deposits (plaque), and trigger microglia to phaocytose the amyloid (Citron, 2010). The second mechanism (Figure B) is a direct interact interaction of amyloid-specific antibodies with the amyloid deposits. The antibodies are able to resolve the in vitro aggregated Aβ, however research is still being done on how the amounts of antibody administered can dissolve the existing insoluble fibrils in the brain (Citron, 2010). A follow-up mechanism was proposed, in which peripheral amyloid-specific antibodies act as a sink (Figure C), and pull soluble Aβ into periphery where it is cleared (Citron, 2010). In vivo studies identified an efficient receptor-mediated transport mechanism for Aβ at the blood brain barrier, where Aβ is transported from CNS to plasma, and from plasma to CNS (Demattos, Bales, Cummins, Dodart, Paul & Holtzman, 2001). Research data suggests that to alter the CNS Aβ levels, increase efflux of Aβ from CNS to plasma and/or decrease efflux of Aβ from plasma to CNS is needed (Demattos et al., 2001). The experiment demonstrated that the Aβ monoclonal antibody 266 (m266) showed affinity to soluble Aβ, and did not bind to plaques (Demattos et al., 2001). This reduced the amyloid levels upon administration. It was concluded that sufficient antibody concentrations were required to produce noticeable levels of cerebrospinal fluid capture needed to capture soluble Aβ, and produce a net flux of Aβ from the CNS to periphery, leading to decreased amyloid levels (Citron, 2010). Although peripheral administration of m266 reduced Aβ deposition, m266 did not bind to the deposits (Demattos et al., 2001). Hence, m266 appears to reduce brain Aβ burden by altering the CNS and plasma Aβ clearance (Demattos et al., 2001).
References
Allan's References References