Table of Contents
Opioids Powerpoint
Opioids
Opioids can be traced back to the Sumerian people who occupied what is modern day Iraq (Brownstein, 1993). They were able to cultivate the therapeutic properties of poppy seeds, and called the drug “gil” which means joy, and the poppy plant “hul gil” which translates to plant of joy (Brownstein, 1993). The drug was used for multiple purposes, including: preventing children from excessive crying, providing people who were suffering a quick and painless death and as a euphoric agent in religious ceremony, whether it was inhaled or ingested (Brownstein, 1993). Historical documents show that as early as the 10th century, opium was being brought over to Europe from the middle east, and by the 16th century drug abuse and addiction was rampant in countries such as England, Germany, and Turkey (Brownstein, 1993). By the early 1800s, a chemist named Serturner was able to isolate the active ingredient in opium, which was called Morphine after the God of dreams, Morpheus. Since this time, scientists have been constantly trying to find new, stronger forms of the drug. The one that has been most commonly talked about as of late is known as Fentanyl (Brownstein, 1993).
Fentanyl
Fentanyl is a synthetic opioid that is 50-100 times more potent than morphine (Fentanyl, 2016). It is an analgesic, meaning that it works to relieve pain. It was invented in the 1960’s to treat chronic pain, particularly in patients with terminal cancer. It was also used as an anaesthetic for individuals going through intense surgery such as cardiac bypass surgery. Fentanyl was a better alternative to morphine which as used at the time, because it provided greater relief from pain and reduced the risk for respiratory depression, which is a leading cause of death from opioid use. The effects include: euphoria, drowsiness, nausea, confusion, sedation and warmth (Fentanyl, 2016).
Epidemiology
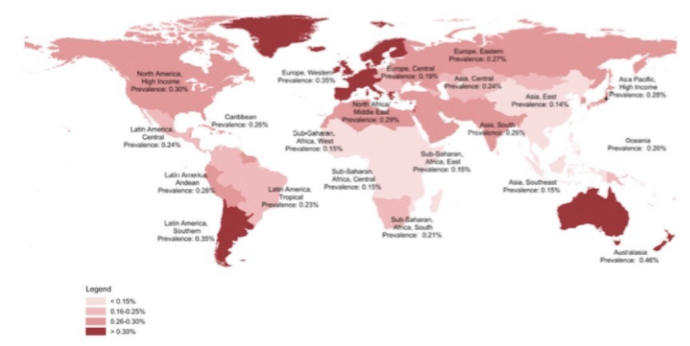
The prevalence of opioid disorders has increased markedly within the past decade. This is largely due to the increase in prescriptions for opioids (Strain, Saxon & Hermann, 2015). An estimated quarter of a billion people used opioids at least once in 2015 (Strain, Saxon & Hermann, 2015). Worldwide, around 35.1 million people have been estimated to use opioids in 2016. Of these individuals, 17.7 million are estimated to have used opiates such as heroin and opium. Global trends indicate that the use of opioids has stabilized at high levels. An area of concern in the past few years has been a significant increase in the non-medical use of prescription opioids. In the US, the rate of prescription opioid use for non-medical purposes was 2.5% in individuals over the age of 12. Moreover, opioid deaths in the US have reached epidemic proportions. Studies show that fatal opioid overdose among injection drug users may be one of the highest causes of preventable death in the US. Nearly half of all US opioid overdose deaths involved a prescription opioid. Among patients prescribed opioids, risk factors for misuse of these medications include a prior history of substance use disorder, younger age, more severe pain as well as concomitant mental disorders (Strain, Saxon & Hermann, 2015).
Mechanism of Action
Fentanyl is a synthetic drug, similar to morphine but 50 to 100 times more potent (Fentanyl, 2016). It is used to treat patients with severe pain (i.e. following surgery) and for individuals with chronic pain who have become desensitized to other pain management medications (Fentanyl, 2016). It is the high potency of fentanyl that increases the risk for overdose, especially for drug addicts who are unaware that a pill may contain fentanyl (Fentanyl, 2016). Fentanyl is an analgesia that has a “rewarding” effect, similar to that of heroin. The effects include euphoria, drowsiness, nausea, confusion, constipation, sedation, tolerance, addiction, respiratory depression and arrest, unconsciousness, coma, and even death (Fentanyl, 2016). Opioids such as fentanyl have an effect on both the central nervous system and the peripheral nervous system (Chahl, 1996).
Pain
Pain is subjective, however it is generally defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage (Chahl, 1996). It is perceived through a combination of sensory and psychological experiences. Pain is experienced when mechanical or thermal stimuli cause increased activity in the primary sensory neurons. This is accompanied by the release of excitatory neurotransmitters in the dorsal horn of the spinal cord, which sends a signal to the central nervous system to indicate pain. The main action of opioids is to inhibit the release of the neurotransmitters at the dorsal horn of the spinal cord to reduce the perception of pain (Chahl, 1996).
Opioid Receptors
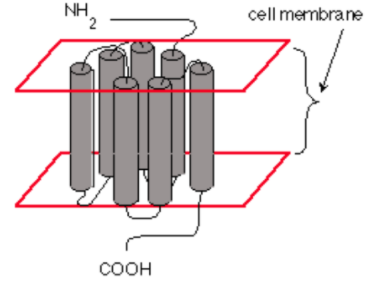
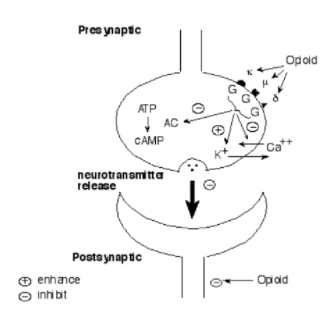
Opioids can act at the pre-synaptic terminal as well as the post-synaptic neuron (Chahl, 1996). Opioids works by binding to specific receptors located on neuronal cell membranes, which are found in areas of the brain that process pain and emotion (Chahl, 1996). There are three types of opioid receptors, mu, delta and kappa. Fentanyl predominantly acts on the mu-opioid receptor of sensory neurons which activates coupled guanine nucleotide binding proteins known as G-protein receptors (Chahl, 1996). GTP is then exchanged for GDP causing a conformational change and activating the effector system (Chahl, 1996). Subsequently, adenylate cyclase is inhibited, which is the enzyme that converts adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP) (Trescot, Datta, Lee & Hansen, 2008). This inhibition decreases the levels of intracellular cAMP, which causes the inhibition of nociceptive (detect harm/pain) neurotransmitters such as substance P, GABA, acetylcholine and noradrenaline (Trescot et al., 2008). The G-protein complex also inactivates calcium channels and activates potassium channels, leading to an outward flow of potassium ions from neurons resulting in hyperpolarization (Trescot et al., 2008). The cumulative result is a decrease in nerve conduction and neurotransmitter release, leading to the inhibition of pain signals (Trescot et al., 2008). In this way, fentanyl increases the patient’s tolerance for pain and decreases the perception of suffering (Fentanyl, 2016). However, the presence of pain may still be recognized (Fentanyl, 2016). Furthermore, opioid receptors are also found in the areas of the brain that control breathing rate (Fentanyl, 2016). High doses of opioids, especially potent opioids such as fentanyl, can cause breathing to stop completely, which can lead to death (Fentanyl, 2016). In this case, naloxone which is an opioid antagonist can reverse the overdose and restore normal respiration (Fentanyl, 2016).
Tolerance and Dependence
Tolerance occurs when there is prolonged exposure to the drug and it means that higher doses of the drug are required to have an effect (Chahl, 1996). This is due to receptor desensitization caused by the uncoupling of the opioid receptors from the G-protein complex, and therefore uncoupling from the effector systems (Chahl, 1996). Dependency accompanies tolerance and can be used interchangeably with addiction. It occurs when the removal of the drug causes withdrawal symptoms (Chahl, 1996). These include stomach cramps, diarrhea, sweating, elevated heart rate and increased blood pressure, irritability, dysphoria, hyperalgesia and insomnia (Chahl, 1996).
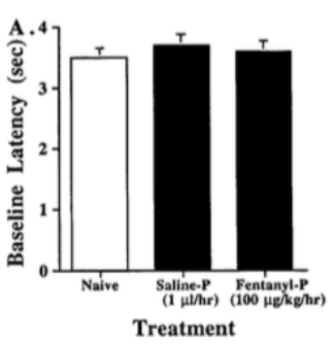
A study completed on neonatal rats tested for the tolerance and dependence effects resulting from continual intravenous fentanyl infusion (Thornton, Wang & Smith, 1997). Rats infused with fentanyl for 72 hours were compared to a control group as well as rats infused with a saline solution (Thornton, Wang & Smith, 1997). Following 72 hours, researchers performed a tail flick test to assay for antinociception, whether the painful stimulus was blocked (Thornton, Wang & Smith, 1997). There was no observed difference between the naïve, saline infused or the fentanyl induced rats (Fig. 1), indicating that fentanyl had a reduced effect and therefore the rats had become tolerant to it (Thornton, Wang & Smith, 1997). The rats were also observed to have become dependent to fentanyl following continual administration of the opioid (Thornton, Wang & Smith, 1997). When these rats were given naloxone, they exhibited a high level of various withdrawal symptoms including, wall climbing behavior, severe tremors (i.e. head turning and shaking) and spontaneous vocalizations (i.e. high pitched screaming) (Thornton, Wang & Smith, 1997).
Adverse Effects of Opioid Use
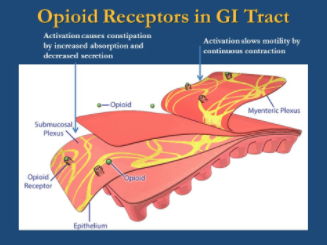
There are various adverse effects associated with long term use of opioids (Voon, Karamouzian & Kerr, 2017). These effects have been shown to target the gastrointestinal, cardiovascular, respiratory and central nervous systems (Voon, Karamouzian & Kerr, 2017). With regards to the gastrointestinal system, constipation is a prevalent effect of chronic opiate therapy (Khansari, Sohrabi & Zamani, 2013). This process depends on the stimulation of μ and κ opioid receptors in the gastrointestinal tract. Studies show that 40-45% of patients on opiate therapy experience constipation. Treatment involves the use of stool softeners and laxatives, which is not always effective. In some cases, constipation can be so severe that patients reduce their medication dosage or discontinue opiate use altogether (Khansari, Sohrabi & Zamani, 2013).
Long term use of opioid therapy has been shown to have deleterious effects on the cardiovascular system (Chen & Ashburn, 2015). When the use of opioid therapy was compared to Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective cyclooxygenase-2 (COX-2) inhibitors, opioid therapy was associated with a 77% increased risk of cardiovascular effects such as heart failure and myocardial infarction (MI) (Voon, Karamouzian & Kerr, 2017). After 30 days, the risk of cardiovascular events was similar across different opioid medications. However, after 180 days of therapy, codeine was associated with a 62% increase in adverse events when compared to hydrocodone (Voon, Karamouzian & Kerr, 2017). Furthermore, studies have shown an increased risk of MI among patients on chronic opioid therapy relative to the general population (Chen & Ashburn, 2015).
With regards to the respiratory system, recent studies have demonstrated an associated between sleep-disordered breathing and chronic opiate use (Voon, Karamouzian & Kerr, 2017). The chronic use of opiates has been shown to be associated with sleep apnea, ataxic breathing and carbon dioxide retention. When analyzing patients undergoing opioid therapy for at least 6 month, the prevalence of sleep-disordered breathing has been found to be as high as 75%. In contrast, the prevalence of sleep apnea in the general population is 3%-20%, where it is found in 30% of patients on chronic methadone therapy (Voon, Karamouzian & Kerr, 2017). Furthermore, a potentially life-threatening side effect of chronic opioid therapy is respiratory depression, bradycardia and hypotension, which occurs in opioid overdose (Boom et al., 2012). The mechanisms are not well understood, however, a physiological tolerance to a certain opioid dose does not necessarily parallel a tolerance to the respiratory effects of opioids (Boom et al., 2012). Moreover, it has been shown that patients prescribed a larger opioid dose experienced a substantially increased overdose risk (Boom et al., 2012).
Opiates active specific receptors along the nervous system (Vella-Brincat & Macleod, 2007). There are three major receptor subtypes, μ, κ, and δ, of which subtype μ seems to be more common. Opioid neurotoxicity is a significant issue, particularly among the elderly. Some of the effects of long term opioid use on the central nervous system (CNS) include dizziness and sedation (Vella-Brincat & Macleod, 2007). Patients on chronic opioid therapy have demonstrated hyperalgesia, which is characterized by excessive sensitivity to pain (Baldini, Korff & Lin, 2012). Furthermore, patients on chronic opioid therapy have been shown to have relatively higher levels of comorbid clinical depression (Baldini, Korff & Lin, 2012).
Medical Use of Opioids
Fentanyl is typically prescribed and administered via two methods: the transdermal patch and intravenous injection. Both are monitored and regulated by healthcare professionals, and are given to patients for specific medical reasons.
Pain Management
Pain is often inadequately addressed and managed in patients, which can in part be attributed to hesitation to employ the use of opioids, including fentanyl, stemming from fear that they will be abused (Joranson et al., 2000). Fentanyl is utilized for the relief of severe chronic pain (such as pain associated with cancer). This drug operates in the brain to modulate how the body feels and responds to pain (Joranson et al., 2000). This can be achieved through the forms of transdermal patch and intravenously (Joranson et al., 2000). The patch form of fentanyl should only be used when absolutely required, and not used in cases of mild pain or pain that is short-term (“Fentanyl Transdermal”, 2018; Joranson et al., 2000). Fentanyl is not suited for occasional or “as needed” use. It may take up to 24 hours before a patient experiences pain relief from fentanyl patches. The patch is usually changed every 72 hours (“Fentanyl Transdermal”, 2018; Joranson et al., 2000).
Intravenously, fentanyl is distributed under the name Sublimaze (Janssen, 2014). It is mainly used for pain management prior to surgery and pain relief after medical procedures. This is always administered by health care professional. The usual dosage is 50 to 100 micrograms (1 to 2 mL) and is given intramuscularly. In elderly and poor-risk patients, a lesser dose of 25 to 50 micrograms (0.5 to 1mL) is recommended. The dose may be re-administered every one to two hours if required. Dosage should of course be adjusted based on each patient’s individual needs, and some of the factors that are used to determine dose are: age, body weight, physical status, underlying pathological condition, use of other drugs, type of anaesthesia, and type of surgical procedure. Due to the many potential side effects, health care professionals monitor patients closely and regularly evaluate vital signs after administering this drug (Janssen, 2014).
Emergence Delirium and Relaxation
Fentanyl can be administered at the end of sevoflurane anaesthesia, normally during surgical procedures, to decrease the incidence and severity of emergence agitation (Janssen, 2014). Emergence agitation in children after anaesthesia has an incidence of up to 80% and is a frequent postoperative issue. The typical behaviours affiliated with emergence agitation are crying, disorientation, excitation, and delirium. Although emergence agitation usually does not result in adverse outcomes, it is still associated with the risk of self-injury and precipitates stress in family members and caregivers (Janssen, 2014). Low doses of fentanyl (1 µg per kg) have been shown to efficiently manage and reduce emergence agitation when given to the patient near the end of anaesthesia (Kim et al., 2012).
Recreational Use of Opioids
Fentanyl is a drug that has a massive abuse potential. Any individual who uses fentanyl that does not have a previous tolerance to opioids is largely at risk. Many first-time recreational users of fentanyl are in drastic danger of overdose. Fentanyl is highly addictive and addictions often develop quickly, which is why prescription fentanyl is so closely monitored by physicians (Lautieri, 2016). Fentanyl has an extreme potency, and is much stronger than other opioids including morphine and oxycodone. On the street, fentanyl often replaces the selling of high-grade heroin (also known as as “China White), and mixed into heroin to magnify the high (Lautieri, 2016). Fentanyl is now frequently being incorporated into other street drugs to elevate potency and addictiveness. Recreational users often consume fentanyl seeking the short-term effects of pain relief, euphoria, and relaxation. Shockingly, over the last decade, the access to recreational drugs, including fentanyl, over the internet has significantly grown and only become easier (Cunningham et al., 2015). According to Cunningham et al. (2015), in a toxicological analysis of a deceased individual by the cause of fentanyl overdose revealed fentanyl concentrations in blood, liver, vitreous fluid, and urine of respectively 235 ng/mL, 2400 ng/g, 131 ng/mL, and 234 ng/mL, which is significantly greater than medical dosing.
Drug Cutting
Drug cutting refers to the addition of different solvents to a pure drug product in order to either reduce the cost of drug production or increase the potency of the drug (Fentanyl, 2016). The materials added to the drug include baking soda, rat poison as well as other drugs such as fentanyl. Additives such as baking soda and rat poison are used to dilute the product which increases profit for the drug seller. The addition of other drugs such as fentanyl is used to increase the potency of the drug, which increases consumer appeal (Fentanyl, 2016).
Drug Trade and Trafficking of Opioids
The “Beekeeper” was considered one of the Canada's most dangerous chemists for many years (CBC, 2017). He was well known for creating many synthetic opioids such as fentanyl (CBC, 2017).
Video: One of Canada’s most prolific chemists, The Beekeeper.
Naloxone
Naloxone is a medication used to treat the effects of opioids. It was patented in 1961 and approved by the Food and Drug Administration for opioid overdoses in 1971 (William, 2013). Naloxone has a few different modes of delivery, it can be administered intravenously, injected into the muscle or sprayed into the nose. The effects of naloxone last from 30 minutes to one hour and because of this, multiple doses must be administered because the duration of most opioids is greater than this (Ngai et al., 1976).
A study conducted by Alexander Y. Walley and his associates wanted to evaluate the impact of state supported overdose education programs. Their aim was to look at the outcome of naloxone distribution programs and the rates of opioid related deaths. The study spanned from 2002 -2009 and concluded that opioid overdose death rates were reduced significantly in communities where the overdose education programs were implemented compared to communities with no intervention (Walley et al., 2013).
Mode of Action
Naloxone is a lipophilic compound that acts as a non-selective, competitive opioid receptor antagonist. The pharmacologically active isomer in naloxone is (-) naloxone, its binding affinity is highest for mu-opioid receptors. Naloxone has a higher binding affinity for the opioid receptor compared to opioids like fentanyl which is what makes it such an effective treatment (Dowling et al., 2008).
Naloxone only acts on opioid receptors, so it will not help with drug overdoses that are not opioids since they do not use the same receptors within the body. If naloxone is administered by itself, it will not cause an effect on the individual due to it being an antagonist (Filizola & Devi, 2012). It will, however, cause the body to not be able to combat pain naturally. The mechanism is not completely understood but researchers believe it is a competitive antagonist because it prevents the action of both endogenous and xenobiotic opiates on the opioid receptors while not producing any effects itself (Sauro & Greenberg, 2018).
Naloxone has no effect on the body if no opioids are present because it binds to the body’s natural opioid receptors. It has been shown to have common side effects with individuals who have opioids in their body which include flushing, dizziness, tiredness, weakness, nervousness, restlessness, fever, nausea, chills, shortness of breath seizures and death. Naloxone has also been shown to block the body’s natural pain lowering endorphins. It is thought that naloxone acts on the same receptors that these endorphins operate on (Cunha, 2016).
Conclusion
Opioids are substances which act on opioid receptors in the body to relieve pain (Voon, Karamouzian & Kerr, 2017). While these drugs have beneficial uses for the relief of chronic pain they have massive abuse potential (Voon, Karamouzian & Kerr, 2017). The abuse of these drugs has lead to an increase in opioid overdosing, particularly in Canada (Fentanyl, 2016). In Ontario there were 336 opioid related deaths from May to July 2017 and 2449 emergency room visits due to overdosing from July to September 2017. Any individual that is using prescribed or street opioids is at risk of having an overdose, however most individuals overdose because they are using street drugs which are laced with potent substances. Street drugs laced with opioids such as fentanyl and carfentanil can be particularly dangerous because they can be fatal even in very small amounts. Individuals using opioids can reduce their risk of overdose by not using the drugs alone and having a naloxone kit available (Fentanyl, 2016).
Moving forward, there is a need for more research on problematic substance use in order to identify ways to mitigate the problem (Fentanyl, 2016). Furthermore, the public needs to be educated on the use of opioids and other legal and illegal drugs (Fentanyl, 2016).
References
1. Baldini, A., Von Korff, M., & Lin, E. H. (2012). A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. The primary care companion to CNS disorders, 14(3).
2. Boom, M., Niesters, M., Sarton, E., Aarts, L., W Smith, T., & Dahan, A. (2012). Non-analgesic effects of opioids: opioid-induced respiratory depression. Current pharmaceutical design, 18(37), 5994-6004.
3. Brownstein, M. J. (1993). A brief history of opiates, opioid peptides, and opioid receptors. Proceedings of the National Academy of Sciences, 90(12), 5391-5393.
4. Chahl, L. A. (1996, July 01). Opioids - mechanisms of action | Australian Prescriber. Retrieved February 22, 2018, from https://www.nps.org.au/australian-prescriber/articles/opioids-mechanisms-of-action
5. Chen, A., & Ashburn, M. A. (2015). Cardiac effects of opioid therapy. Pain Medicine, 16(suppl_1), S27-S31.
6. Cooper, A. J., Willis, J., Fuller, J., Benecke, H., Leighton-Scott, J., Andersohn, F., … & Knaggs, R. D. (2017). Prevalence and Incidence Trends for Diagnosed Prescription Opioid Use Disorders in the United Kingdom. Pain and therapy, 6(1), 73-84.
7. Cunha, J. (2016). Narcan(Naloxone Hydrochloride Injection) side effects drug center. RxList. Retrieved from https://www.rxlist.com/narcan-side-effects-drug-center.htm#overview
8. Dowling, J., Isbister, G. K., Kirkpatrick, C. M. J., Naidoo, D., & Graudins, A. (2008). Population pharmacokinetics of intravenous, intramuscular, and intranasal naloxone in human volunteers. Therapeutic Drug Monitoring, 30(4), 490–496. http://doi.org/10.1097/FTD.0b013e3181816214
9. Fentanyl. (2016, June 03). Retrieved February 22, 2018, from https://www.drugabuse.gov/publications/drugfacts/fentanyl
10. “Fentanyl Transdermal : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing.” WebMD, WebMD, www.webmd.com/drugs/2/drug-6253/fentanyl-transdermal/details.
11. Filizola, M., & Devi, L. A. (2012). Structural biology: How opioid drugs bind to receptors. Nature, 485(7398), 314–317. http://doi.org/10.1038/485314a
12. Janssen. (2014). SUBLIMAZE Injection. http://www.janssen.com/australia/sites/www_janssen_com_australia/files/prod_files/live/sublimaze_pi.pdf
13. Joranson DE, Ryan KM, Gilson AM, & Dahl JL. (2000). Trends in medical use and abuse of opioid analgesics. JAMA, 283(13), 1710–1714. https://doi.org/10.1001/jama.283.13.1710
14. Khansari, M., Sohrabi, M., & Zamani, F. (2013). The useage of opioids and their adverse effects in gastrointestinal practice: a review. Middle East journal of digestive diseases, 5(1), 5.
15. Kim, M. S., Moon, B. E., Kim, H., & Lee, J. R. (2012). Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. British journal of anaesthesia, 110(2), 274-280.
16. Lautieri, M. “The Effects of Fentanyl Use.” DrugAbuse.com, 29 Jan. 2016, drugabuse.com/library/the-effects-of-fentanyl-use/.
17. Cunningham, S. M., Haikal, N. A., & Kraner, J. C. (2016). Fatal Intoxication with Acetyl Fentanyl. Journal of Forensic Sciences, 61, S276–S280. https://doi.org/10.1111/1556-4029.12953
18. Ngai, S. H., Berkowitz, B. A., Yang, J. C., Hempstead, J., & Spector, S. (1976). Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology, 44(5), 398–401. http://doi.org/10.1097/00000542-197605000-00008
19. Roy, G. (2017, September 15). Opioid-related overdose figures show grim reality of Canadian epidemic. Retrieved February 21, 2018, from https://www.theglobeandmail.com/news/national/opioid-related-overdose-figures-show-grim-reality-of-canadian-epidemic/article36257932/
20. Sauro, M. D., & Greenberg, R. P. (2018). Endogenous opiates and the placebo effect. Journal of Psychosomatic Research, 58(2), 115–120. http://doi.org/10.1016/j.jpsychores.2004.07.001
21. Stanley, T. H. (1992). The history and development of the fentanyl series. Journal of pain and symptom management, 7(3), S3-S7.
22. Strain, E., Saxon, A. J., & Hermann, R. Opioid use disorder: Epidemiology, pharmacology, clinical manifestations, course, screening, assessment, and diagnosis. UpToDate. 2015.
23. The CBC. (2017, March 09). Retrieved February 21, 2018, from http://www.cbc.ca/firsthand/episodes/unstoppable-the-fentanyl-epidemic
24. Thornton, S. R., Wang, A. F., & Smith, F. L. (1997). Characterization of neonatal rat morphine tolerance and dependence. European Journal of Pharmacology,340(2-3), 161-167. doi:10.1016/s0014-2999(97)01434-9
25. Trescot, A. M., Datta, S., Lee, M., & Hansen, H. (2008). Opioid pharmacology. Pain physician, 11(2 Suppl), S133-53.
26. Vella-Brincat, J., & Macleod, A. D. (2007). Adverse effects of opioids on the central nervous systems of palliative care patients. Journal of pain & palliative care pharmacotherapy, 21(1), 15-25.
27. Voon, P., Karamouzian, M., & Kerr, T. (2017). Chronic pain and opioid misuse: a review of reviews. Substance abuse treatment, prevention, and policy, 12(1), 36.
28. What is Heroin Cut With? Mixing Agents, Substitutes & Adulterants. (n.d.). Retrieved February 22, 2018, from https://americanaddictioncenters.org/heroin-treatment/cut-with/
29. William, Y. (2013). Jack Fisman Dies at 83; Saved Many From Overdose. Saved Many From Overdose. NY Times. Retrieved from http://www.nytimes.com/2013/12/15/business/jack-fishman-who-helped-develop-a-drug-to-treat-overdoses-dies-at-83.html?_r=0