Table of Contents
History
Attention deficit hyperactivity disorder, more commonly known as ADHD, is a neurobehavioral disorder usually diagnosed in children. Originally, ADHD was called hyperkinetic impulse disorder and was not recognized as a mental disorder until the late 1960s (Lange, Reichl, Lange, Tucha, & Tucha, 2010). In 1902, a British pediatrician by the name of Sir George Still described some children to have “an abnormal defect of moral control'. Although these children did not control their behavior like a 'typical' child, they were still intelligent. In 1936, Dr. Charles Bradley discovered that children prescribed a drug called Benzedrine showcased large improvements in their behaviour and performance in school. Unfortunately, Bradley's colleagues didn't recognize the importance of his findings until many years later (Lange, Reichl, Lange, Tucha, & Tucha, 2010).
When the Diagnostic and Statistical Manual of Mental Disorders (DSM) was first published, ADHD was not included. The second edition of the DSM included hyperkinetic impulse disorder but the third edition, released in 1980, changed the name to attention deficit disorder (ADD). This is because not all scientists believed that hyperactivity was a common symptom (Lange, Reichl, Lange, Tucha, & Tucha, 2010). In 1987, the third version of the DSM decided to combine the three common symptoms (inattentiveness, impulsivity and hyperactivity) and changed ADD to ADHD. Finally, the 2000 edition of the DSM established three subtypes of ADHD as:
1. Combined type ADHD
2. Predominantly inattentive type ADHD
3. Predominantly hyperactive-impulsive type ADHD
During the late 1990s, the number of ADHD cases began to significantly increase. This was mainly attributed to the fact that doctors were able to diagnose ADHD more efficiently. Currently, scientists are focusing on identifying the causes of ADHD but we have many medications that treat the disorder with long-term benefits (Lange, Reichl, Lange, Tucha, & Tucha, 2010).
Epidemiology
ADHD is known as a highly familial disease and is one of the most heritable psychiatric disorders (Salamanca, 2014). These is no global consensus on the prevalence of ADHD but the prevalence rates vary depending on multiple factors, such as:
1. Age
2. Gender
3. Presentation of Symptoms
Age
Initially, ADHD was thought to only inhibit children and decline during maturation into adulthood. Now, ADHD is known to persist into adulthood in approximately 50-66% of individuals. A study conducted worldwide showed that about 1.8-6.4% of pre-school children had ADHD while 5.29-7.1% of individual under the age of 18 have ADHD (Salamanca, 2014).
Gender
There is a lot of evidence that suggests that ADHD is more prevalent in males compared to females. Although, the European ADORE (Attention-deficit/hyperactivity Disorder Observational Research in Europe) study suggested that there is no difference in symptomatology. Regardless, most research is focused on males with ADHD research in females is still limited (Salamanca, 2014).
Presentation of Symptoms
In a worldwide analysis of 86 studies, it was determined that predominantly in attentive types of ADHD is the most common subtype across all samples. The only exception was pre-school children that mainly exhibited predominantly hyperactive-impulsive type (Salamanca, 2014).
Neurobiology
Many studies have shown various structural, functional and chemical correlations with ADHD in children, adolescents and adults (Greenberg, 2015).
Structural
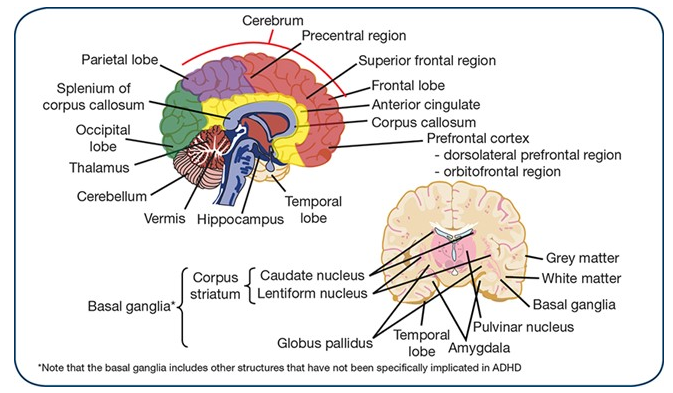
Several brain imaging studies have shown structural abnormalities with ADHD patients, such as (Greenberg, 2015):
- Delayed cortical development
- Cortical thinning
- Reductions in grey and white matter volumes
- Reductions in posterior interior vermis, splenium of the corpus callosum, right cerebral, right caudate, right globus pallidus, right anterior frontal region, cerebellum, temporal lobe and pulvinar
Figure 1: This image highlights the areas of the brain that show structural abnormalities in patients with ADHD (Greenberg, 2015)
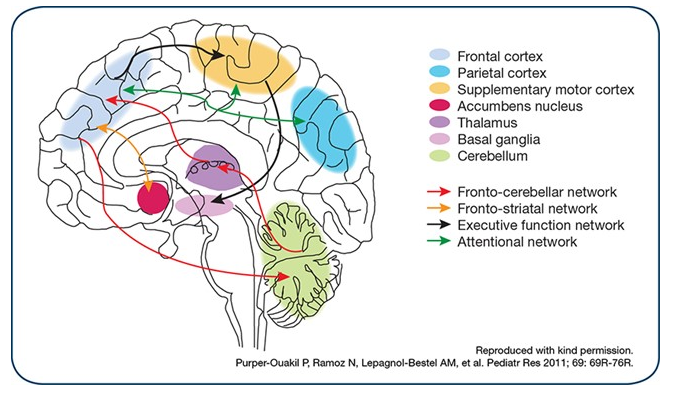
Functional
There are significant activation abnormalities with patients with ADHD, such as reductions in various frontal regions of brain, such as (Greenberg, 2015):
- Anterior cingulate
- Dorsolateral prefrontal and inferior prefrontal cortices
- Basal ganglia
- Thalamus
- Parietal cortex
Figure 2: This image highlights the areas of the brain that show functional abnormalities in patients with ADHD (Greenberg, 2015)
Chemical
Children and adolescents with ADHD have delayed maturation of specific dopaminergic neural pathways. In addition, these individuals also have an imbalance of dopamine and noradrenaline. Dopamine and noradrenaline have been shown to influence impulsivity while dopamine alone influences inattention. Current research is focusing on other signalling systems such as glutamate regulation and polymorphisms in serotonin transporter (Greenberg, 2015).
Diagnosis
Diagnostic Criteria from DSM-5
According to Diagnostic and Statistical Manual (DSM) 5, the 3 types of attention deficit/hyperactivity disorder (ADHD) are predominantly inattentive, predominantly hyperactive/impulsive, and combined (American Psychiatric Association, 2013). The specific criteria for attention-deficit/hyperactivity disorder are as follows:
Inattentive
This must include at least 6 of the following symptoms of inattention that must have persisted for at least 6 months to a degree that is maladaptive and inconsistent with developmental level (American Psychiatric Association, 2013):
- Often fails to give close attention to details or makes careless mistakes in schoolwork, work, or other activities
- Often has difficulty sustaining attention in tasks or play activities
- Often does not seem to listen to what is being said
- Often does not follow through on instructions and fails to finish schoolwork, chores, or duties in the workplace (not due to oppositional behavior or failure to understand instructions)
- Often has difficulties organizing tasks and activities
- Often avoids or strongly dislikes tasks (such as schoolwork or homework) that require sustained mental effort
- Often loses things necessary for tasks or activities (school assignments, pencils, books, tools, or toys)
- Often is easily distracted by extraneous stimuli
- Often forgetful in daily activities
Hyperactivity/Impulsivity
This must include at least 6 of the following symptoms of hyperactivity-impulsivity that must have persisted for at least 6 months to a degree that is maladaptive and inconsistent with developmental level (American Psychiatric Association, 2013):
- Fidgeting with or tapping hands or feet, squirming in seat
- Leaving seat in classroom or in other situations in which remaining seated is expected
- Running about or climbing excessively in situations where this behavior is inappropriate (in adolescents or adults, this may be limited to subjective feelings of restlessness)
- Difficulty playing or engaging in leisure activities quietly
- Unable to be or uncomfortable being still for extended periods of time (may be experienced by others as “on the go” or difficult to keep up with)
- Excessive talking
- Blurting out answers to questions before the questions have been completed
- Difficulty waiting in lines or awaiting turn in games or group situations
- Interrupting or intruding on others (for adolescents and adults, may intrude into or take over what others are doing)
Other
As in the DSM-IV, the presence of sufficient inattentive and/or hyperactive impulsive symptoms is only the initial criteria that must be met for ADHD to be diagnosed. Additional diagnostic criteria, and modifications that have been made to these and incorporated into the DSM-5, are presented below (American Psychiatric Association, 2013):
- Onset is no later than age 12 years
- Symptoms must be present in 2 or more situations, such as school, work, or home
- The disturbance causes clinically significant distress or impairment in social, academic, or occupational functioning
- Disorder does not occur exclusively during the course of schizophrenia or other psychotic disorder and is not better accounted for by mood, anxiety, dissociative, personality disorder or substance intoxication or withdrawal
In addition, attention-deficit/hyperactivity disorder is specified by the severity based on social or occupational functional impairment: mild (minor impairment), moderate (impairment between “mild” and “severe”), and severe (symptoms in excess of those required to meet diagnosis; marked impairment (American Psychiatric Association, 2013).
Diagnostic Tools
Diagnostic Forms & Scales
Diagnostic tools are used to determine if an individual has ADHD and which treatments are appropriate for their conditions. Physicians use scales and checklists obtained from parents, teachers and other regarding symptoms and functioning in various settings. It is important to note that the use of scales and checklists are only one component of the evaluation. Other components include a medical examination and interview (CHADD, 2016).
Conners Comprehensive Behavior Rating Scale
The Conners Comprehensive Behavior Rating Scale (Conners CBRS) is a questionnaire that assesses children from ages 6-18 who may potentially have ADHD. It observes behaviors, emotions, academic and social problems in children (Conners, 2013). Three forms assess behavior, one for the parents, one for the teachers and a self-report conducted by the child if between the ages of 8-18. The Conners CBRS is made up of many components, including DSM-5, symptoms scales, content scales, other clinical indicators, critical items and impairments items (Conners, 2013). This diagnosis tool aims to assist with the diagnostic process, identifies and qualifies children for special education, assists with the development of intervention and treatment plans and evaluates its effectiveness (Conners, 2013).
Barkley Home & School situations questionnaire
The Barkley Home Situations Questionnaire (HSQ) and Barkley School Situations Questionnaire (SSQ) were developed by Dr. Russell Barkley. According to the DSM-5, symptoms of ADHD must be visible in at least two environments thus these questionnaires are designed to gather information from both parents and teachers (Bailey, 2016). The HSQ measures how symptoms of ADHD disrupt regular home situations such as watching TV or conducting chores (Bailey, 2016). Parents must rate problems on a scale from 1-9 in sixteen different areas (Bailey, 2016). The SSQ measures 12 common school situations such as listening to instructions, sitting at desks, paying attention in the classroom etc. Physicians use these questionnaires along with complete evaluations to determine the correct diagnosis. Results from these questionnaires along with the results from the DSM-5 will help the physician determine if a child has ADHD. (Bailey, 2016)
Wender Utah Rating Scale
The Wender Utah Rating Scale created by Paul Wender is another questionnaire used to assess adults with ADHD. The questionnaire consists of 61 questions about the patient’s childhood, which must be answered on a scale of 0-4. Twenty-five of the questions are associated with ADHD. According to the Wender Utah Rating Scale, a score of 46 or higher indicates patients having ADHD during their childhood (Ward, 1993).
Imaging
In most cases physicians use behavior to diagnose patients with ADHD but when the physician requires confirmation of diagnosis or the diagnosis is questionable more testing is approached. In these cases the use of imaging techniques are used as the next approach to diagnosis patients. However it is important to note, these imaging tools cannot be used alone to make a final diagnosis. It must be combined with other test results such as the behavioral questionnaires.
Single Photon emission computed tomography (SPECT)
SPECT is the most common testing technique to measure for ADHD. SPECT is a 20 minute neuroimaging technique that measures the blood flow within the brain. It shows regions in the brain that are active (hot) and quiescent (cold) while patients complete various tasks (Sherman, 2015).
Quantitative electroencephalography (qEEG)
qEEG measure brain activity by observing distinctive brain patterns. Patients wear an electrode-studded cap and gel is applied to the head to conduct electrical impulses (Sherman, 2015). The brain waves show physicians abnormalities in the frontal area of the brain. ADHD patients show excess slow waves while others may have way too fast-wave activity (Sherman, 2015). This testing helps physicians determine which medication to prescribe and which will be most effective.
Other
Other imaging tests that are currently being used to assist in the diagnosis of ADHD are positron emission tomography (PET) and magnetic resonance imaging (MRI). These brain scans reveal the significant sizes in the brain. However studies have shown that MRI scanning is not the best diagnostic tool to prove a patient has ADHD (Cai et al, 2015).
Symptoms
Many symptoms that occur can be seen in the DMS-5 criteria scale however other symptoms are appearance, mood, speech and thought process and cognition. Cognition being a common symptom includes having difficulty with calculation tasks and recent memory tasks. For adults, symptoms may be reckless driving, poor listening skills, marital problems, trouble relaxing and lateness.
Pathophysiology and Etiology
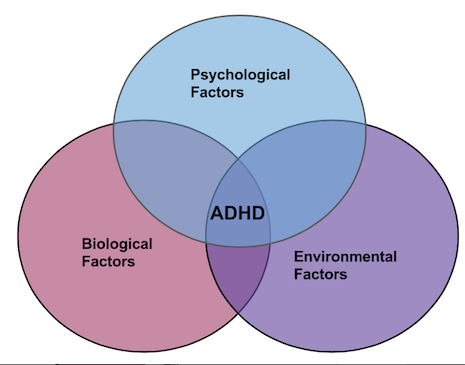
The biopsychosocial model of ADHD portrays a broad view which attributes the disorder outcomes to the interaction of three factors: biological, psychological, and environmental.
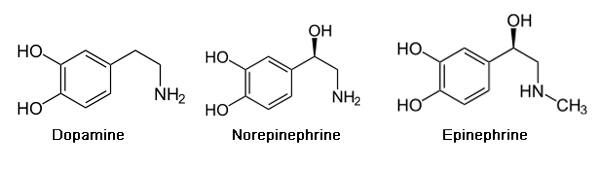
Pathophysiology of ADHD is unclear and the roots have yet to be discovered. Drugs used to treat ADHD have led to hypothesizing the involvement of certain areas in the brain involved in attention have some sort of neural transmission deficits. The underlying brain regions are thought to be involved distributed over various brain regions as opposed to a single area. Indicated though PET scan imaging, the most notable structural and functional alterations are in parietal lobes and cerebellum (Cherkasova & Hechtman, 2009). Specifically, the neurotransmitters dopamine and norepinephrine are associated with ADHD.
Psychological Factors
Temperamental Factors
Temperament is the behavioral style that individuals consistently exhibit as a response to their environments (Foley, McClowry & Castellanos, 2008). It is evident early in life, relatively stable across time and various situations, biologically-driven and genetically-linked. For children with ADHD, three aspects of temperament are evident (Foley, McClowry & Castellanos, 2008): 1. Neuroticism (intensity and frequency of the expression of negative response to stress); 2. Conscientiousness (persistence in task performance); and 3. Extraversion/Activity (highly outgoing/overactive).
A study by Jensen and Rosen (2004) performed a study involving parent-ratings, where mothers of children with ADHD were asked to rate the intensity and frequency their child’s emotional responses. They found that children with ADHD were rated to be significantly more emotionally reactive to both immediate and future events compared to children without ADHD. In addition, children with ADHD showed a greater response to negative emotional events (both in immediate and future time periods) compared to positive emotional events. However, when rating the responses to the consequences of their behavior, children with ADHD were rated as less emotionally reactive than children without ADHD (Jensen & Rosen, 2004).
Hoza et al. (2001) investigated academic task persistence of boys with ADHD compared to boys without ADHD. The methodology involved a success-failure manipulation with find-a-word puzzles. Results show that boys with ADHD solved fewer test puzzles, had a higher frequency of quitting on the task, and found fewer words on a generalization task. In addition, boys with ADHD were observed to be less effortful and less cooperative in performing the task compared to the control group (Hoza et al., 2001).
Many children with ADHD (particularly the hyperactive type) are often described as ‘extreme extraverts’ or ‘over-active’ (Shaw, 2011). High levels of extraversion in ADHD has been linked to the insufficient amounts of dopamine in the brain regions linked to the reward-system. This chronic underarousal is hypothesized to be the underlying reason why these children subconsciously aim to compensate for this deficit by seeking more rewards through highly overt behavior. This excessive activity might also be due to their lack of motor inhibition; these children have trouble controlling movements due to deficiencies in gross and fine motor movements. Through measurements that track control of hand movements, children with ADHD appear to have tremendous difficulty in controlling unnecessary movements during motor tasks like simple finger-sequencing. As a result, these children show too much unnecessary movements, as well as difficulty in motor coordination and control, and have difficulty with performing a variety of tasks that involve motor skills, such as handwriting (Shaw, 2011).
Attention
There are three components of attention (Huang-Pollock, Nigg & Halperin, 2006): 1. *Selective attention* (the process of attenuating to only one thing at a time despite many non-relevant, distracting stimuli in the environment); 2. *Attentional orienting in space* (for instance, turning one’s attention towards a light stimulus flashing in one direction; and 3. *Arousal and vigilance* (the ‘readiness’ to pay attention in the event that a stimulus appears, or a state of ongoing arousal and alertness).
In individuals with ADHD, there are no apparent deficits in selective attention and attentional orienting in space. However, the arousal and vigilance components of attention are dysfunctional. In other words, these individuals have no impairments in arousal responses to stimuli, but rather they have deficits in their ‘preparedness’ in paying attention to these stimuli. When presented with a stimulus, they will not respond with the typical arousal responses as seen in control groups because of their low levels of alertness to pay attention to these stimuli (Huang-Pollock, Nigg & Halperin, 2006).
Response Inhibition
Response inhibition is the cognitive process of inhibiting oneself from making a dominant response when it is not appropriate in the situation (an example is a child with ADHD who persistently moves around the classroom despite being told by the teacher to sit quietly, and the rest of the children are sitting down). This process is impaired in individuals with ADHD. A study by Wodka et al. (2007) examined response inhibition in children with ADHD using three ‘go/no-go tests’: one with high cognitive demand, one with low cognitive demand, and one that is motivation-linked (have rewards and response costs). In ‘go/no-go’ tasks, test subjects are presented with a continuous stream of stimuli and they are asked to perform a binary decision on each stimulus. One of the stimuli requires participants to make a motor response (‘go’), whereas the other requires participants to withhold a response (‘no-go’). Accuracy and reaction times are measured for each event, and the dominant response is established by presenting a higher proportion of the ‘go’ stimuli, where the subjects are supposed to make the motor response (Wodka et al., 2007).
Results show that compared to control groups, children with ADHD made more errors in each of the simple, cognitive and motivation-linked go/no-go tests, i.e. over the course of several trials, these children made the ‘go’ response for the ‘no-go’ stimuli. These results show that the inability to inhibit the dominant response appears to be a primary deficit that is observed, even when the executive function demands of tasks are minimal, and even in the presence of rewards and motivational factors for performing the task correctly (Wodka et al., 2007).
Working Memory
Working memory is the component of short-term memory that is concerned with the immediate, conscious perceptual and linguistic processing (Barnett, Maruff & Vance, 2005). This process is impaired in ADHD, with huge deficits specifically in visuospatial working memory. A study by found that ADHD individuals demonstrated subtle but significant impairment in the performance of visuospatiial memory tasks. The memory impairment was delay-independent, which suggest dysfunction of the encoding rather than the retrieval phase of visuospatial memory. In other words, this may reflect attentional deficits rather than being due to the dysfunction of the explicit memory system (Barnett, Maruff & Vance, 2005).
Biological Factors
Family Studies
Increased rates of ADHD are often found in children of parents and siblings with ADHD.These correlating high risks indicates strong familial risk factors involved in the development of ADHD, whether that be genetic or environmental. Familial study findings have indicated additive genetic effects of ADHD within the range of 75-90%. This supports the understanding of some sort of underlying trait, most likely influenced by several rather than a single gene (Levy et al, 1997). In some family studies, it has also been found that low birth weight has a significant influence in twins (Freitag & Retz, 2011).
Adoption Studies
Adoption studies seek to identify the degree of influence on ADHD symptoms from environmental factors, although they can also be used to support the degree of influence of biological factors. This is done by comparing the prevalence of the disorder between the affected individual and their adoptive and biological relatives. If a disorder were to be environmentally influenced, then the individual should have a greater rate of correlation of symptoms with adoptive family. However, the findings of these studies can be limited due to many study designs not controlling for certain risk factors, such as the presence of maternal smoking in the adopted child’s biological parent (Freitag & Retz, 2011).
Twin Studies
Twin studies can provide insight into the extent of which a trait is due to genetic factors versus environmental and nurture factors. These studies are done by comparing the occurrence ADHD in monozygotic (MZ) twins, who share identical genomes, to dizygotic (DZ) twins, who share approximately half of their genes. Twin and family studies have shown a 60-80% concordance rate was determined (Freitag & Retz, 2010). Despite many twin studies having been done over the years with differing study designs, all results seem to be conclusively consistent. The nearly unanimous results have identified that individual differences in ADHD symptoms can be largely attributed to genetic influences, with an average heritability of .73 across all studies (Willcutt et al, 2010). However, most studies have been done on children with ADHD, identified as being between the ages of 8 and 18, and there is not much information currently on these twin studies having been done on adults (Freitag & Retz, 2011).
Candidate Gene Studies
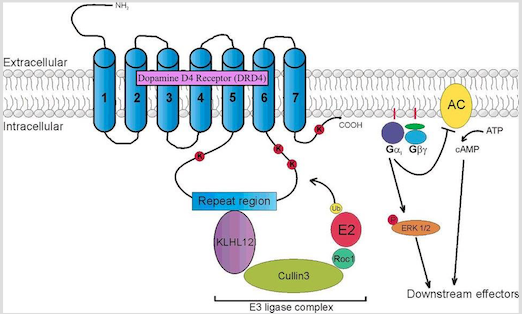
Several quantitative genetic studies have suggested that genetic influences contribute significantly to the pathogenesis and development of ADHD (Gizer, Ficks & Waldman, 2009). Over the past 15 years, substantial efforts have been made in order to identify genes involved in the etiology of the disorder. Given that abnormalities in the dopamine neurotransmission has been suggested to underlie the etiology of ADHD, the genes that code for dopamine receptors have been identified as candidate loci for ADHD (Gizer, Ficks & Waldman, 2009). Two of the most studied genes are the the Dopamine D4 receptor gene (DRD4) and the Dopamine transporter gene (DAT1).
DRD4 gene
DRD4 is a G protein-coupled receptor belonging to the dopamine D2-like receptor family, which act to inhibit adenylyl cyclase (Gizer, Ficks & Waldman, 2009). The DRD4 gene has been mapped to position 15.5 of the p-arm of chromosome 11. It is mostly expressed in the frontal lobe regions of the brain, such as the orbitofrontal cortex and anterior cingulate, thus playing a role in the regulation of higher-level cognitive functions such as reward anticipation, decision-making, impulse control, and emotion. Association studies have linked this gene to the personality trait of novelty seeking, and has been likened to the high levels of impulsivity and excitability seen in the diagnosis of ADHD. In addition, DRD4 knockout mice have been shown to exhibit a heightened response to cocaine and metamphetamine compared to control groups, as seen through increases in their locomotor activity. The most widely studied DRD4 polymorphism is the 48-bp variable number of tandem repeats (VNTR) in exon 3 of the gene. This VNTR has been shown to play a role in secondary messenger systems (i.e. cyclic AMP) and in responses to antipsychotic medication such as clozapine (Gizer, Ficks & Waldman, 2009).
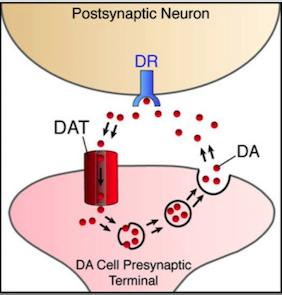
DAT1 gene
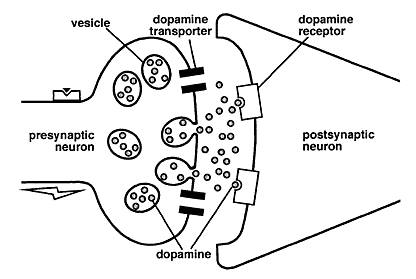
The DAT1 gene is located in position 15, in the p-arm of chromosome 5 (Gizer, Ficks & Waldman, 2009). The gene codes for a carrier protein that is responsible for the recycling of dopamine from the synaptic cleft back to the presynaptic neuron. This protein is widely distributed in the corpus striatum and the nucleus accumbens, and represents the basis of dopamine regulation in these brain areas. Stimulant medications for ADHD such as methylphenidate have been shown to inhibit the function of DAT1, thereby increasing the levels of available dopamine in the synapse and decreasing the manifestation of ADHD symptoms. In addition, mice that were genetically modified to have a higher density of the DAT1 gene presented the phenotypic behaviors of ADHD, such as increased motor activity compared to wild-type controls. The most widely investigated polymorphism of DAT1 is a VNTR sequence in the 3’ untranslated region of the gene. Several studies show that the dopamine transporter availability and binding potential are influenced by genotype at this VNTR (Gizer, Ficks & Waldman, 2009).
Neurological Basis
The neurological underpinnings of ADHD are evident in electroencephalogram (EEG) and neuroimaging studies (Gustafsson et al., 2000). First, results show that about 20% of individuals diagnosed with ADHD have abnormally low EEG, which is consistent with the hypothesis that these individuals have lower levels of dopaminergic activity in the limbic/reward system areas of the brain. Hyeractivity in overt behavior is a compensation for this underarousal. Drug treatments to stimulate and counteract this underarousal, and raise brain wave activity, is thought to prevent ADHD individuals from engaging in hyperactive symptoms. Second, there is a higher amount of cortical slow wave activity in ADHD individuals, which is consistent with the underarousal of the cortex. Third, ADHD individuals have weaker measured evoked response potentials (ERP’s) to stimuli. In other words, these subjects do not respond as quickly as would be expected to the presence of stimuli in the environment. This is consistent with the underarousal of the cortex, as well as the impairment of the arousal and vigilance functions of the attentional process seen in these individuals. Fourth, there is a lower amount of blood flow in the caudate and frontal lobes and a higher blood flow in the primary sensory cortices, especially the temporal and occipital lobes, in patients with ADHD (Gustafsson et al., 2000). A new interesting finding has shown that individuals with ADHD show a significantly reduced asymmetry in the sizes of the right and left prefrontal cortices (Schneider et al., 2006). In the general population, the right hemisphere of the brain is typically larger in size, whereas in individuals with ADHD, the size difference is not as significant. The implications of this reduced asymmetry on ADHD symptomatology is still being debated upon. In addition, about 90% of individuals with right cortical lesions exhibit ADHD symptoms, so the right hemisphere of the brain is of particular interest when studying the etiology of the disease. Lastly, post-mortem studies show that three brain structures are smaller in ADHD patients compared to the general population: the basal ganglia, the corpus callosum, and the vermis of the cerebellum. The exact mechanisms and relation to ADHD symptoms are still being investigated (Schneider et al., 2006).
The catecholamine hypothesis of ADHD states that the three main neurotransmitters that play a role in ADHD are the catecholamines: norepinephrine, serotonin, and dopamine, and that there is a significant impairment in the dynamics of these catecholamines in the neural synapse, which is correlated with the symptomatology of the disease (Pliszka, McCracken & Maas, 1996). The central norepinephrine system may be dysregulated in ADHD, such that the system does not sufficiently prime the cortical posterior attention system to external stimuli in the environment. Also, reduced activity of norepinephrine in the reticular formation puts the brain in a ‘sleepy’ state. The effective mental processing of information involves the anterior executive system (i.e. the prefrontal cortices) which largely depend on dopaminergic circuits. Dopamine is also the basis of regulating the reward/gratification of the brain. Again, these are deficient in the brain of an individual with ADHD. Serotonin has an inhibitory effect on the other two catecholamines. It is hypothesized that ADHD individuals have too much serotonin activity, leading to an excessive deactivation of norepinephrine and dopamine (Pliszka, McCracken & Maas, 1996).
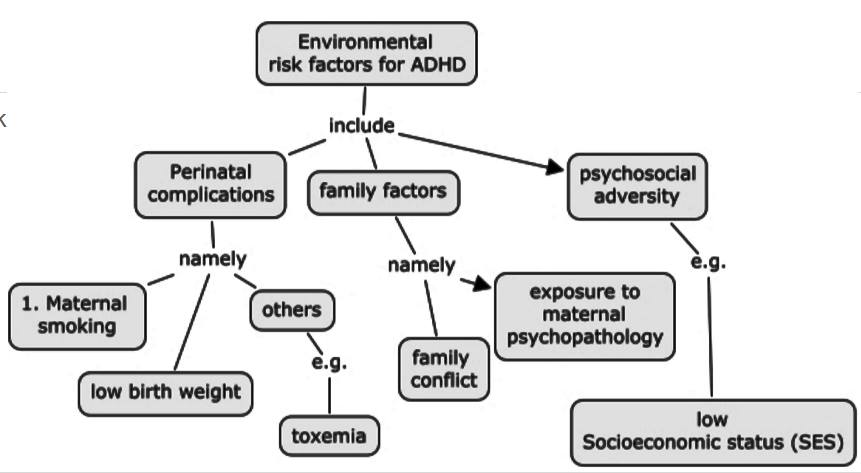
Environmental Factors
Various environmental factors are thought to contribute as risk factors to the development of ADHD such as including food additives/diet, pre- and postnatal toxin exposure or mercury or lead, cigarette and alcohol exposure, maternal smoking during pregnancy, and low birth weight. Many recent studies have specifically examined the relationships between ADHD and these extraneous factors (Banerjee et al, 2007). However, it is to be noted that these studies show that these environmental risk factors and potential gene–environment interactions increase the risk for developing ADHD. In addition to this, even if one does not have a high expression of the candidate genes of risk, but were exposed to an environmental risk factor, there is also a risk for developing ADHD.
Treatment
Methylphenidate (e.g. Ritalin)
Methylphenidate (MPH) is the most commonly prescribed drug for the treatment of ADHD (Volkow et al, 2002). It is a stimulant drug that blocks dopamine and norepinephrine transporters in the brain, thereby increasing the levels of dopamine in the brain (Volkow et al, 2002).
Though the exact mechanism of action is widely unknown, it may help increase attention and decrease impulsiveness and hyperactivity (FDA, 2013). Methylphenidate potentiates, or strengthens, dopaminergic signalling by increasing the effect of dopamine cell firing on the concentration of dopamine in the synaptic cleft (Tripp & Wickens, 2008).
The Dopamine Transfer Deficit (DTD) theory suggests that there are specific alterations in the magnitude and timing of this anticipatory dopamine cell firing in children with ADHD (Tripp & Wickens, 2008). This anticipatory dopaminergic cell firing is important for behavioural reinforcement in learning (Tripp & Wickens, 2008). The DTD theory predicts that for children with ADHD, there is a dopamine transfer dysfunction. Specifically, the phasic dopamine cell response to the cue that predicts reinforcement is reduced in amplitude to the point of being ineffective (Tripp & Wickens, 2008).
Consistent with the DTD theory, it is beneficial to block the dopamine transporter, DAT, so there is less dopamine reuptake and more dopamine present in the synaptic cleft (Tripp & Wickens, 2008). This type of drug treatment should not be used for children under six years of age because it has not yet been studied for this age group (FDA, 2013). Some side effects of Ritalin include, headache, decreased appetite, stomach ache, nervousness, trouble sleeping and nausea.
Dexmethylphenidate
Most cases of ADHD persist into adulthood. This dug is a safe and effective treatment for ADHD among children and adults (Spencer et al, 2007).
Its mechanism of action is more known and is similar to methylphenidate. This drug binds to and blocks the presynaptic dopamine transporter in the basal ganglia, resulting in an increased amount of dopamine in the synaptic cleft (Spencer et al., 2007).
In preclinical studies, the drug raised extracellular levels of dopamine (Spencer et al., 2007). This form of treatment is available in tablet form and should be taken 2-3 times daily due to the short-half life (Spencer et al., 2007).
It was noted that dopamine levels were elevated in the nucleus accumbens as well (Bymaster et al., 2002). The nucleus accumbens is involved in the reward circuit, thereby increasing the susceptibility of abuse of psychostimulants; stimulants are considered a Schedule II drug meaning that there is a high potential for abuse (Allen et al., 2005).
The downside is that some children reported to feel embarrassed at school when taking the medication and would often skip it (Tripp & Wickens, 2008).
Another pitfall is that individuals with ADHD are often forgetful and may miss a dosage of their medication and thus impairs the efficacy of the drug (Tripp & Wickens, 2008).
In a specific study, the only side effects experienced were a dry mouth (Spencer et al., 2007).
Adderall
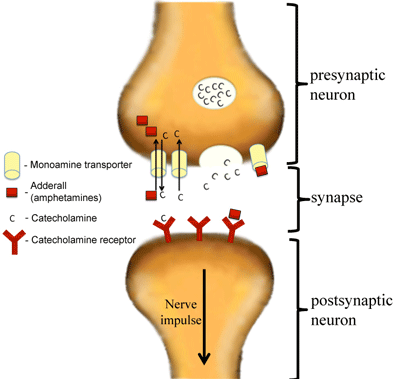
Adderall is a chemical compound that is made up of a mixture of the amphetamine salts that is used to enhance attention (Sherzada, 2012). The function of Adderall is to block the reuptake of norepinephrine, dopamine, and serotonin into the presynaptic neuron and increase the release of these monoamines into the synaptic space (Sherdzada, 2012). Monoamines include catecholamines (epinephrine, norepinephrine and dopamine). Dopaminergic activity is enhanced, again by binding to DAT, the dopamine transporter, and inhibiting the reuptake of dopamine to increase levels of dopamine in the synaptic cleft (Sherzada, 2012).
Normally, neurotransmitters such as dopamine are stored in vesicles in the presynaptic neuron, and are released when triggered by an action potential. Amphetamine enters the presynaptic neuron and forces dopamine to be released from the vesicles and into the synaptic cleft (Sherzada, 2012). Essentially, the dopamine transporter DAT is working in reverse, so as to release dopamine rather than reuptake it.
This reduces the symptoms of inattention, impulsivity and hyperactivity. Some possible side effects in children and adolescents include loss of appetite, insomnia, abdominal pain, nausea and vomiting (Sherzada, 2012). Adults may experience dry mouth, loss of appetite, insomnia, headache, weight loss, nausea, anxiety, agitation, dizziness, tachycardia, diarrhea, asthenia, and urinary tract infections (Sherzada, 2012).
CBT
Cognitive-behaviour therapy (CBT) was initially targeted for the treatment of depression but it is now used to treat adults with ADHD (Rostain and Ramsey, 2006). The focus of CBT is to change a person’s negative, problematic thoughts and beliefs to create downstream changes in emotions and behaviors since this model suggests that dysfunctional thinking plays a central role in the development and/or maintenance of clinical symptoms (Rostain and Ramsey, 2006). CBT along with the use of medication showed improvements in the symptoms of ADHD as well as improvements on measures of depression (Rostain and Ramsey, 2006). The goal of CBT is to provide structure, education and copping strategies for managing ADHD while also providing personal support (Rostain and Ramsey, 2006). It is thought that medications aid with the neurobiological aspects of ADHD but CBT teaches individuals how to actively manage symptoms increasing compensatory strategies in organizing and planning, coping with distractibility, and enhancing optimal thinking strategies (Safren et al., 2005).
Non stimulants
Non stimulants are also prescribed to treat ADHD. An example is Atomoxetine, which has high selectivity for noradrenergic reuptake transporters and low selectivity for dopaminergic transporters in the nucleus accumbens, thereby increasing norepinephrine specifically in the prefrontal cortex (Allen et al., 2005). This is important because it does not disrupt sleep as severely; a side effect of stimulants (Allen et al., 2005). In addition, the low selectivity for dopamine means that the nucleus accumbens is not affected as severely as with stimulants, so there is less potential for abuse of this drug (Allen et al., 2005).
Conclusions and Future Implications
Current Research
Current research suggests there are fewer indications of ADHD in children whose mothers took vitamin D supplements during pregnancy. There is a lot of research suggesting that that vitamin D is essential for the neuroanatomical development of a fetus (Mossin et al., 2016). The precise mechanism through which higher vitamin D concentrations protect against ADHD symptoms remain a mystery. It is hypothesized that the protective property of vitamin D works through its anti-inflammatory abilities thereby inhibiting harmful environmental triggers related to potential neurodevelopmental abnormalities (Mossin et al., 2016). This was tested in vitamin D deficient pregnant mice were associated with increased glucocorticoid exposure in the fetus, which impaired normal brain development (Mossin et al., 2016). Therefore, suffice vitamin D concentrations may potentially protect the brain from developing abnormalities leading to ADHD (Mossin et al., 2016).
Future Research
Although there is currently a lot of research on AHDH, there are still some gaps (Vaidya, 2011). Namely, there needs to be more studies which include an appropriate amount of females to examine any gender differences (Vaidya, 2011). Additionally, it is important to keep in mind that the use of longitudinal studies using functional brain imaging are necessary to examine varying performance levels at different developmental stages (Vaidya, 2011). These factors may be crucial to further understand the underlying mechanisms of ADHD.
References
5 Most Common Adderall Addiction Signs. (2016). Amphetamines.com. Retrieved 24 October 2016, from http://amphetamines.com/types/adderall/5-common-adderall-addiction-signs/
Allen, A., Kurlan, R., Gilbert, D., Coffey, B., Linder, S., & Lewis, D. et al. (2005). Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology, 65(12), 1941-1949. http://dx.doi.org/10.1212/01.wnl.0000188869.58300.a7
Bailey, E. (2016). Rating Scales for Diagnosing ADHD. Retrieved October 19, 2016, from http://www.healthcentral.com/adhd/treatment-253024-5.html
Banerjee, T. D., Middleton, F. and Faraone, S. V. (2007), Environmental risk factors for attention-deficit hyperactivity disorder. Acta Pædiatrica, 96: 1269–1274. doi:10.1111/j.1651-2227.2007.00430.x
Barnett, R., Maruff, P., & Vance, A. (2005). An investigation of visuospatial memory impairment in children with attention deficit hyperactivity disorder (ADHD), combined type. Psychological medicine, 35(10), 1433-1443.
Bymaster, F. (2002). Atomoxetine Increases Extracellular Levels of Norepinephrine and Dopamine in Prefrontal Cortex of Rat A Potential Mechanism for Efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology, 27(5), 699-711. http://dx.doi.org/10.1016/s0893-133x(02)00346-9
Cai, W., Chen, T., Szegletes, L., Supekar, K., & Menon, V. (2015). Aberrant cross-brain network interaction in children with attention-deficit/hyperactivity disorder and its relation to attention deficits: A multisite and cross-site replication study. Biological psychiatry.
CHADD. (2016). Rating Scales and Checklists. Retrieved October 25, 2016, from http://www.chadd.org/Understanding-ADHD/For-Professionals/For-Healthcare-Professionals/Clinical-Practice-Tools/Rating-Scales-and-Checklists.aspx
Cognitive Behavioural Therapy at Trio Psychology. (2016). Triopsychology.com. Retrieved 24 October 2016, from http://triopsychology.com/cbt
Conners, K. C. (2013). Conners Comprehensive Behavior Rating Scales. Retrieved October 19, 2016, from http://www.mhs.com/product.aspx?gr=edu Foley, M., McClowry, S. G., & Castellanos, F. X. (2008). The relationship between attention deficit hyperactivity disorder and child temperament. Journal of Applied Developmental Psychology, 29(2), 157-169.
Freitag, C. M., Retz, (2010). Attention-Deficit Hyperactivity Disorder (ADHD) in Adults. Key Issues in Mental Health. 176, 38–57
Gizer, I. R., Ficks, C., & Waldman, I. D. (2009). Candidate gene studies of ADHD: a meta-analytic review. Human genetics, 126(1), 51-90.
Gustafsson, P., Thernlund, G., Ryding, E., Rosén, I., & Cederblad, M. (2000). Associations between cerebral blood‐flow measured by single photon emission computed tomography (SPECT), electro‐encephalogram (EEG), behaviour symptoms, cognition and neurological soft signs in children with attention‐deficit hyperactivity disorder (ADHD). Acta Paediatrica, 89(7), 830-835.
Hoza, B., Waschbusch, D. A., Owens, J. S., Pelham, W. E., & Kipp, H. (2001). Academic task persistence of normally achieving ADHD and control boys: Self-evaluations, and attributions. Journal of Consulting and Clinical Psychology, 69(2), 271.
Huang-Pollock, C. L., Nigg, J. T., & Halperin, J. M. (2006). Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology, 20(4), 420.
Jensen, S. A., & Rosen, L. A. (2004). Emotional reactivity in children with attention-deficit/hyperactivity disorder. Journal of attention disorders, 8(2), 53-61.
Levy, F., Hay, D.A., McStephen, M., et al.(1997). Attention deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child & Adolescent Psychiatry, 36:737– 744.
MEDICATION GUIDE RITALIN. (2013). Retrieved 14 October 2016, from http://www.fda.gov/downloads/drugs/drugsafety/ucm089090.pdf
Mossin, M., Aaby, J., Dalgard, C., Lykkedegn, S., Christesen, H., & Bilenberg, N. (2016). Inverse associations between cord vitamin D and attention deficit hyperactivity disorder symptoms: A child cohort study. Australian & New Zealand Journal Of Psychiatry. http://dx.doi.org/10.1177/0004867416670013
Parents Know Your Rights About ADHD & Child Mental Disorders | CCHR International. (2012). CCHR International. Retrieved 24 October 2016, from https://www.cchrint.org/issues/childmentaldisorders/
Pedersen, N. L., McClearn, G. E., Plomin, R. and Nesselroade, J. R. (1992), Effects of early rearing environment on twin similarity in the last half of the life span. British Journal of Developmental Psychology, 10: 255–267. doi:10.1111/j.2044-835X.1992.tb00576.x
Pliszka, S. R., McCracken, J. T., & Maas, J. W. (1996). Catecholamines in attention-deficit hyperactivity disorder: current perspectives. Journal of the American Academy of Child & Adolescent Psychiatry, 35(3), 264-272.
Rostain, A. & Ramsay, J. (2006). A Combined Treatment Approach for Adults With ADHD–Results of an Open Study of 43 Patients. Journal Of Attention Disorders, 10(2), 150-159. http://dx.doi.org/10.1177/1087054706288110
Safren, S., Otto, M., Sprich, S., Winett, C., Wilens, T., & Biederman, J. (2005). Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behaviour Research And Therapy, 43(7), 831-842. http://dx.doi.org/10.1016/j.brat.2004.07.001
Shaw, G. (2011, June). Neurology Now. Retrieved October 18, 2016, from https://patients.aan.com/resources/neurologynow/index.cfm?event=home.showArticle
Schneider, M., Retz, W., Coogan, A., Thome, J., & Rösler, M. (2006). Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)—a neurological view. European archives of psychiatry and clinical neuroscience, 256(1), i32-i41.
Sherman, C. (2015). Can Brain Scans Help Diagnose ADHD? Retrieved October 19, 2016, from http://www.additudemag.com/adhd/article/783-3.html
Sherzada, Awista (2012) “An Analysis of ADHD Drugs: Ritalin and Adderall,” JCCC Honors Journal: Vol. 3: Iss. 1, Article 2. Available at: http://scholarspace.jccc.edu/honors_journal/vol3/iss1/2 Spencer, T., Adler, L., McGough, J., Muniz, R., Jiang, H., & Pestreich, L. (2007). Efficacy and Safety of Dexmethylphenidate Extended-Release Capsules in Adults with Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 61(12), 1380-1387. http://dx.doi.org/10.1016/j.biopsych.2006.07.032
The Brain—Lesson 3—Drugs Change the Way Neurons Communicate (Page 1 of 2). (2016). Science.education.nih.gov. Retrieved 24 October 2016, from https://science.education.nih.gov/supplements/nih2/addiction/guide/lesson3-1.html
Tripp, G. & Wickens, J. (2008). Research Review: Dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal Of Child Psychology And Psychiatry, 49(7), 691-704. http://dx.doi.org/10.1111/j.1469-7610.2007.01851.x
Vaidya, C. (2011). Neurodevelopmental Abnormalities in ADHD. Behavioral Neuroscience Of Attention Deficit Hyperactivity Disorder And Its Treatment, 49-66. http://dx.doi.org/10.1007/7854_2011_138
Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002; 6 Suppl 1:S31-43.
Ward, M. F. (1993). The Wender Utah Rating Scale: An Aid in the Retrospective. Am J Psychiatry, 1(50), 885.
Willcutt, E. G., Pennington, B. F., Duncan, L., Smith, S. D., Keenan, J. M., Wadsworth, S., … Olson, R. K. (2010). Understanding the complex etiologies of developmental disorders: Behavioral and molecular genetic approaches.Journal of Developmental and Behavioral Pediatrics : JDBP, 31(7), 533–544. http://doi.org/10.1097/DBP.0b013e3181ef42a1
Wodka, E. L., Mark Mahone, E., Blankner, J. G., Gidley Larson, J. C., Fotedar, S., Denckla, M. B., & Mostofsky, S. H. (2007). Evidence that response inhibition is a primary deficit in ADHD. Journal of clinical and experimental neuropsychology, 29(4), 345-356.