Table of Contents
Kidney Transplants
General Information
Kidneys
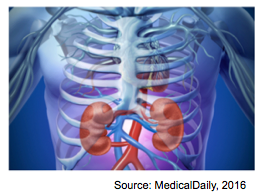
Figure 1: Anatomical Location of Kidneys
</style>
The kidneys are two-bean shaped organs located at the back of the abdominal cavity (also known as the retroperitoneal space)[15]. The kidney is a vital organ that carries out numerous essential functions in our body that are required to sustain life. There are three main functions that the kidneys are involved in: an excretory function, homeostatic function and endocrine function. In particular, the kidneys are responsible to filter blood and remove waste products. The kidneys can filter about 180 L of plasma per day. This means that your entire body gets filtered approximately 20 to 25 times a day[15]! Kidneys also play a role in maintaining our internal balance. For instance, the kidneys regulate water balance and acid-base balance, in addition to blood pressure levels. Kidneys also play a huge role in producing, activating, and secreting essential hormones and vitamins in the body such as vitamin D, as well as proteins such as erythropoietin (EPO). EPO is required for the production of red blood cells[15].
Kidney Disease
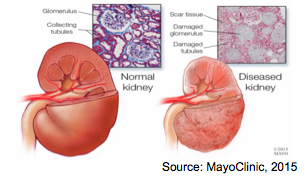
Figure 2: Healthy Kidney vs. Diseased Kidney
</style>
Consequently, damage to the kidneys results in decreased levels of kidney function. Kidney damage and kidney disease may not be easily identifiable in individuals without undergoing specific diagnostic tests. For this reason kidney disease is often referred to as a 'silent' disease. Kidney damage can be an acute or chronic problem. Chronic kidney disease, commonly referred to as CKD, is the gradual loss of kidney function over a period of time (months, years)[4]. In order for an individual to be diagnosed with CKD they will need to have a gradual loss of kidney function over a minimum period of at least three months. Further, CKD is diagnosed in 5 stages. Each stage is characterized by a marker of kidney function called the estimated glomerular filtration rate (eGFR). A decrease in eGFR levels corresponds to decreasing levels of kidney function. The last stage of kidney disease, stage five, is called end-stage renal disease (ESRD) or end-stage kidney failure (ESKF). At this stage the eGFR is less than 15 mL/minute. In normal healthy individuals, eGFR would be greater than 90 mL/min[4].
Prevalence of Kidney Disease
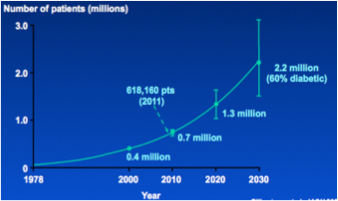
Figure 3: Projected Growth of CKD in the US Population
[15]
</style>
Kidney damage, and in particular, kidney failure is becoming an increasing issue, especially in North America. The number of individuals with CKD is growing and continues to grow each year. According to Collins et al. (2003) the projected growth rate of CKD is 5% per year in the United States. These rates are comparable to the Canadian population. In fact, the number of Canadians with kidney failure have tripled over the last 20 years[15]. This constant rise in the prevalence of chronic kidney disease (and kidney failure) is partly due to the aging population. However, rising obesity rates, as well as rising rates of diabetes and hypertension are also responsible for this increase. In fact, the most common causes of CKD are history of diabetes and hypertension. Other causes of renal failure include glomerular diseases such as glomerulonephritis, and the use of certain medications and toxins including tobacco[15].
Renal Replacement Therapy
Individuals with end-stage kidney failure will require renal replacement therapy. There are two primary treatments for renal replacement therapy: dialysis and renal transplants[15]. Dialysis is a treatment that removes wastes and excess fluids from your blood. There are two types of dialysis treatments: hemodialysis, which cleans the blood through an artificial kidney hooked up to a machine, and peritoneal dialysis, which removes waste products and excess fluids by using the body's peritoneal as a filter. On the other hand, a kidney transplant is an operation to replace a damaged kidney with a donated kidney. The best and most effective replacement therapy would be kidney transplants[15].
Kidney Transplantation
Types of Kidney Donors
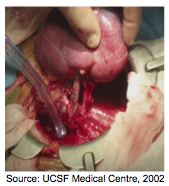
Figure 4: Kidney Transplant
</style>
There are two types of kidney donors (transplants): deceased-donor kidney transplant and living-donor kidney transplant[15]. Living-donor transplants are generally better for the recipient. These individuals have to wait less time for a donor, regain normal kidney functions faster than deceased-donor kidney transplant recipients, and regain normal kidney functions faster. In fact, living-donor recipients have a 90-95% success rate in the first year in comparison to a 85-90% success rate in deceased-donor recipients. Similarly, living-donor kidneys last approximately 15-20 years in comparison to deceased-donor kidneys that last 10-15 years [15].
Procedure
Figure 5: Anatomical Location of a Transplanted Kidney
</style> Kidney transplants are a common procedure in medical practice nowadays. The first kidney transplant in Canada took place in 1950[4]. The technical aspects of the procedure have not greatly changed since the 1970s. A kidney transplant is a heterotopic transplant. This means that the native or diseased kidney is not removed during the transplant and is left in place. The donor kidney is implanted in the pelvic area below the native kidneys. Donor kidneys are one of the least sensitive organs with respect to organ preservation and ischemia tolerance levels. In fact, donor kidneys can last for about 24 to 36 hours prior to transplantation[2]. They are kept in preservation fluids and ice to reduce tissue damage and prevent ischemia.
A typical kidney transplant is about two to three hours on average. The recipient is monitored in the hospital for three to seven days on average, patients who acquire complications will usually stay longer. The donor kidneys may take three to fifteen years to reach normal kidney functioning levels; living-donor transplants are usually on the lower end of this spectrum[15].
https://www.youtube.com/watch?v=ou8CC4XN9wk
Advantages
Kidney transplants are usually the best course of treatment for renal failure, especially in comparison to dialysis treatment. Dialysis can put a huge strain on the body and the individual's lifestyle. Unfortunately, dialysis only carries out ten percent of the functions that a normal, healthy kidney would. On the contrary, individuals who receive a kidney transplant have a longer life expectancy, have less expenses to deal with, and face fewer complications[15]. Individuals living with a kidney transplant generally have a better quality of life with less diet-restrictions. They feel healthier, more energetic, and have less nutrient deficiency problems.
Disadvantages
There are some contraindications with a renal transplant; these include: pulmonary, cardiac and hepatic insufficiency, history of metastatic cancer, and morbid obesity. Emotional and psychological well-being of the recipient is also taken into consideration prior to a transplant. Currently, there are more than 3,000 Canadians waiting for a kidney transplant[13]. Difficulties finding a match, and the long waiting lists are two primary concerns with regards to kidney transplants. Canada's transplant system puts “geography in front of needs” of patients, putting the more vulnerable patients at risk. In fact, it can four to six years on average for an individual to be matched with a kidney in Ontario and British Colombia [12]. The possibility of rejection post-transplant is also a concern, however, rejection is less of a problem nowadays due to immunosuppressant medications.
Kidney Transplant Rejection
Minimizing Risks of Rejection
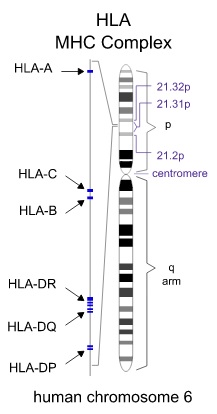
Human Leukocyte Antigen (HLA) is the human version of the commonly studied Major Histocompatibility Complex (HMC), highly involved in immunological reactions. This HLA molecule is expressed on the surface of all cells in the human body, presenting randomly generated peptides from the interior[20]. This allows for the immune system to monitor whether cells are healthy or infected, as well as whether an immune response needs to be mounted. To prevent the immune system from recognizing the newly transplanted graft as foreign, the donor and recipient both have their HLA genes checked using high-resolution DNA typing techniques[20]. The HLA system has loci across chromosome 6, expressing 3 classes of HLA[20]. When matching HLA between the donor and recipient, HLA –A, –B, and –DR are checked for similarities[20]. There are a vast number of alleles for these genes (>800 type –A and –B, >400 type –DR), adding to the complexity of matching donors and recipients appropriately to minimize risk of rejection[20].
Figure 6: Chromsome 6 Expresses 3 Classes of HLA
</style>
Internationally, HLA matching between donors and recipients differ by the acceptability of mismatches[20]. Countries with organ transplant systems regulate the amount of HLA mismatch they deem is acceptable in order for the transplant to be viable[20]. In the United States, because the majority of organ donors belong to Caucasian ancestry, the HLA alleles are skewed towards certain populations[20]. This puts recipients of differing ancestral backgrounds at a disadvantage when an organ transplant is vital for their health, such as in the case of end-stage renal diseases[20].
Maximizing the similarity of HLA between the donor and recipient not only minimizes the risks of transplant rejection, but also minimizes the probability of graft versus host disease[20]. This is a case when the immune cells present from the transplanted graft recognize the new host as foreign, and proceed to mount an immunological attack[20]. HLA matching also presents dualistic effects; matching may improve the outcome of the kidney transplant, but may also enhance the cellular immune mechanisms leading to further graft dysfunction[20].
Allorecognition
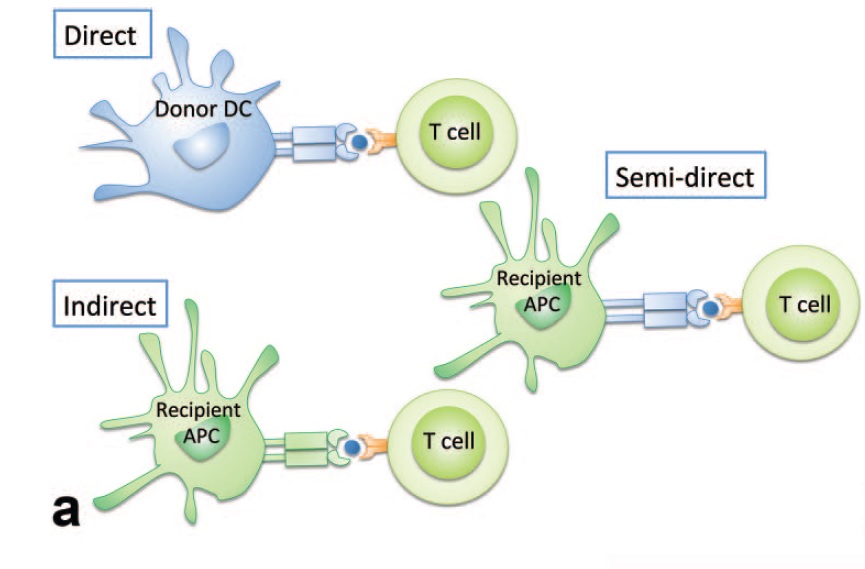
In many cases of renal transplantation, the recipient’s immune system is able to recognize that the transplanted kidney is a foreign organ, by a process termed allorecognition. This allows the immune system to prime T-cells to recognize and launch an immune response against the new organ [3]. There are 3 main ways via which allorecognition occurs:
- Direct allorecognition occurs when donor dendritic cells (or other antigen-presenting cells) from the graft present a graft peptide to T-cells of the recipient’s immune system. This usually results in immediate rejection, but the response becomes less likely to occur over time due to the decrease in donor dendritic cell count [3].
- Indirect allorecognition occurs when recipient antigen-presenting cells display a graft antigen to the recipient T-cells. This is the most common occurrence that is responsible for chronic rejections [3].
- Semi-direct allorecognition occurs when recipient antigen-presenting cells “capture” donor MHC complexes with an antigen from the graft and presenting it to T-cells of the recipient’s immune system. The function of semi-direct allorecognition has not yet been elucidated [3].
Figure 7: Allorecognition in Renal Transplants
</style>
Types of Rejection
Before performing a kidney transplantation, physicians ensure that the donor kidney is a match to the recipient [20]. Often times, even when an HLA antigen match has occurred, the recipient’s immune system may still detect the donor organ has foreign; triggering a severe immune response. There are three types of rejection:
- Hyper Acute Rejection: This is the most rapidly elicited form of kidney transplant rejection. Hyper acute rejection occurs a few minutes after the transplant has taken place and is the result of mismatched ABO blood types; triggering a humoral immune response. For example, this would occur if the recipient had type A blood and the donor had type B blood. In this case, the donor organ tissue must be removed right away to prevent death of the recipient [3].
- Acute Rejection: Acute rejection takes at least a week to occur and is modulated by cytotoxic T cells; signifying the involvement of the immune system. In this case, the recipient has circulating antibodies in their blood before the transplant occurs. The T cells produce cytokines that then recruit other inflammatory mediators leading to the eventual death of the kidney tissue [3].
- Chronic Rejection: Chronic rejection describes long-term dysfunction of the donated organ [3]. In most patients, chronic rejection takes several months to elicit. It is the most prevalent cause of renal dysfunction, usually characterized by a gradual loss of kidney function, coupled with hypertension and excess protein in the urine. Chronic rejection initially develops in tissue grafts that are prone to continuous damage as a result of indirect recognition of alloantigens [3]. In this case, the immune modulators destroy the endothelium of blood vessels, depleting the donor organ tissue of blood.
Signs of Rejection:
- Fatigue
- Fever or flu-like symptoms
- Elevated protein levels in urine (proteinuria)
- Elevated granzyme and perforin levels in urine
- Elevated creatine levels
Mechanisms of Rejection
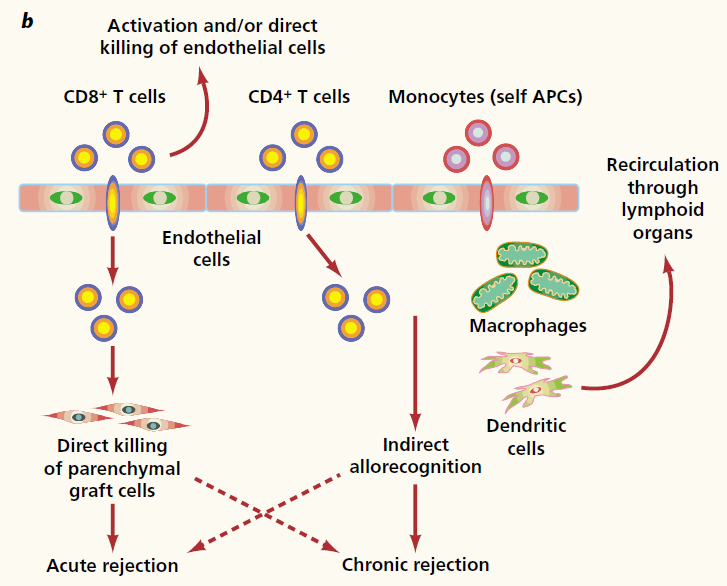
Overall, allorecognition leads to the priming of T-cells, activating them to respond to the presenting antigen. In most cases, dendritic cells or other antigen-presenting cells are exposed to these peptides first, resulting in their activation and migration to the thymus. In the thymus, the antigen is presenting via MHC to T-cells, allowing for their maturation and ability to launch an immune response [20]. Recently, it has been elucidated that T-cells do not necessarily need to become activated at the Thymus, but can also be activated directly at the graft via interactions with the endothelial cell lining of the transplant [20]. The microenvironment where T-cells become activated leads to the differentiation of T-cells, varying in their cytokine signatures and functionalities (CD8+ T-cells versus CD4+ T-cells). This differentiation depends on the expression of a master transcription factor, determining the final subset of cytotoxic T-cells (CD8+) and T-helper cells (CD4+). During renal transplants, there are many factors that can affect the microenvironment post-transplant [20]:
Figure 8: Mechanisms of Rejection
</style>
- Immune status of the recipient at the time of transplant
- Degree of ischemia-reperfusion
- Degree of donor-recipient HLA mismatch
- Current immunosuppressive regime
CD8+ T-cells, also known as cytotoxic T-cells, are typically activated after forming a three-cell cluster between itself, a CD4+ T-cell, and an antigen-presenting cell [20]. It can directly destroy graft cells by expressing perforin to disrupt their membrane and injecting granzyme in to the cell to destroy crucial proteins via the release of cytotoxic granules. FasL released by the cytotoxic T-cell can bind to FasR on the target cell, resulting in the triggering of apoptosis of the graft cell by triggering various cascades [20]. CD4+ T-cells release cytokines that result in inflammation, and can attract other immune cells towards the location of the graft. Recent studies indicate that CD4+ T-cells can result in graft rejection by themselves, although the exact mechanism is not clear [20].
Tolerance
Pharmacotherapy
The aim of pharmacotherapy in kidney transplantation is to avoid graft rejection, minimize side effects and prevent metabolic complications. The leading drugs that allow for successful graft transplants are immunosuppressants. Immunosuppressants fall under a class of drugs that decrease the strength of the immune system. Immunosuppressants are also known as anti-rejection drugs. In kidney transplant, anti-rejection drugs suppress the body's ability in detecting a foreign organ and consequently rejecting it [9].
Immunosuppressants are currently the leading cause of success in acute allograft acceptance. There are two classes of anti-rejection drugs used, depending on the time surpassed since the transplantation procedure: induction drugs and maintenance drugs [9].
Induction drugs are used at the time of the transplantation. The ultimate aim of induction drugs is to avoid acute rejection during the early stages of post-transplatnation by administering a high potency of immunosuppression. Induction immunosuppressive agents include monoclonal agents (such as alemtuzumab, daclizumab, muromonab-CD3) or polyclonal agents. This includes antithymocyte globulin [rabbit]) antibodies or antithymocyte globulin [equine] [6].
Maintenance drugs are used for long-term medication after the procedure. Such drugs include calcineurin inhibitors, corticosteroids, mTor inhibitors and anti-proliferative agents. It is common for an immunosuppression regime to include a combination of multiple drugs [14].
Corticosteroids
Corticosteroids, such as oral prednisolone and IV methylprednisolone, can be used as induction and maintenance drugs. These drugs halt cytokine production and vasoactive substances. These immunosuppressants are alkylating agents, which interfere with DNA replication by cross-linking nucleotides. In addition, corticosteroids act as glucocorticoid receptor agonists. Prednisolone is metabolized in the liver and then excreted as an inactive metabolite by the kidney. The common interactions with corticosteroids include P450 inhibitors [5].
Mammalian Target of Rapamycin (mTOR) Inhibitors
Sirolimus (a rapamycin) is a microbial product of a soil fungus, Streptomyces hygroscopicus, located on the Easter Island. This agent is used for both chronic rejection and maintenance immunosuppression. The mode of action includes preventing IL-2 activation and proliferation, however it does not inhibit calcineurin [7].
Due to the prolonged half-life, multiple drug interactions are quite possible. Concomitant use with strong CYP3A4/P-gp inducers or inhibitors fluctuates the sirolimus concentrations. When cyclosporine is used simultaneously with mTOR inhibitors, there is an increase in the maximum concentration of the drug (Cmax) and area-under-the-curve (AUC) for both compounds. Thus, if both drugs are to be administered, it is recommended to have a 4 hour gap between the administration of cyclosporine and mTOR inhibitors.
Adverse effects from the use of mTOR inhibitors include hyperlipidemia, thrombocytopenia, anemia, pneumonitis, oral ulcers, and diarrhea. These agents can also hinder the healing process of wounds and dehiscence production of lymphoceles. When used concomitantly with calcineurin inhibitors, sirolimus heightens the nephrotoxicity of calcineurin [1].
Calcineurin Inhibitors
Calcineurin Inhibitors (CNIs) are commonly known as the golden standard of care in modern immunosuppression. Calcineurin is a protein phosphatase that activates the T-cells by cleaving off a phosphate group. As a result, inhibitors of calcineurin inactivate the proliferation of T-cells. The two popular CNIs used commonly for the prevention of allograft rejections are Cyclosporine and Tacrolimus [13].
Cyclosporine
Figure 9: Cyclosporine from a Fungal Origin
</style>
The calcineurin inhibitor, cyclosporine, has been employed as an immunosuppressant in transplantation for around 4 decades. It is also used for both induction and maintenance immunosuppression. The molecule comprises a 11 amino acid polypeptide chain from a fungal origin, which inhibits calcineurin phosphatase and T-cell activation through dephosphorylation of inactive nuclear factor of activated T-cells [13]. Thus, it prevents IL-2 via calcineurin inhibition production. The inhibition of IL-2 activation cycle prevent the proliferation of T-cells and thus rendering an effective adaptive immune response. The adverse side effects of cyclosporin include nephrotoxicity, nausea, diarrhea, vomiting, hyperkalemia, and hypomagnesemia [13].
Tacrolimus
Tacrolimus prevents the production of IL-2 through calcineurin inhibition by binding to the tacrolimus binding protein (TBP). This macrolide antibiotic is active against T-helper cells. The tacrolimus FKBP12 active complex inhibits calcineurin with a stronger potency than the corresponding cyclosporine complex. This drug is used particularly for maintenance immunosuppression and during the refractory rejection under cyclosporine-based therapy (known as rescue therapy). Adverse effects are similar to the calcineurin inhibitor, cyclosporine, but with a lower occurrence of hypertension, hyperlipidemia, hirsutism, gum hyperplasia and skin changes. However, it is reported that Tacrolimus has a lower occurrence of acute-rejections and less-pronounced adverse effects. In addition, Tacrolimus can cause reverse alopecia [13].
Future Treatments
Rituximab
A new drug that is being investigated recently is Rituximab, which effectively depletes B cells and decreases the concentration of antibodies. Rituximab binds to CD20 at the precursor and mature B cell surface, thus inhibiting B-cell proliferation and causing cellular apoptosis. B-cells are responsible for producing antibodies; therefore the inhibition of B-cell proliferation prevents antibodies from attacking antigens (and rejecting the kidney) [2].
Intravenous Immunoglobulin
IVIG (intravenous immunoglobulin) causes alloantibody inhibition through the induced apoptosis of B cells. IVIG blocks T cell and B cell activation through Fc receptor-mediated interaction with antigen-presenting cells.
3D-Prints of Kidneys
Figure 10: 3D Print Technology for Customizing Organs
</style>
An exciting form of tolerance to kidney transplant rejections are 3D-Prints of Kidneys, using the host's cells. The goal of 3D-Prints is to construct organs with the patient’s cells. Through this approach, graft rejections will not be an issue (as the organ is constructed through the host's cells) and the use of immunosuppressants will not be required. [18]
The steps of 3D-Prints include the following:
1. Obtain biopsy of the replaced organ.
2. Isolate cells of regenerative capabilities and keep within a mixture of nutrients and oxygen.
3. Place the mixture in a printer cartridge. The printer cartridge shall “print” the organ of desire.
4. Customize the organ based on anthropometric measurements of the patient (through medical scans).
3D-Prints is a relatively new application that is being used to print many organs such as the heart, kidneys and other crucial organs [18].
References
[1] Araki, Motoo, et al. “Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs.” Transplantation 81.3 (2006): 335-341.
[2] Becker, Y. T., Becker, B. N., Pirsch, J. D., & Sollinger, H. W. (2004). Rituximab as treatment for refractory kidney transplant rejection. American Journal of Transplantation, 4(6), 996-1001.
[3] Briscoe, D. M., & Sayegh, M. H. (2002). A rendezvous before rejection: where do T cells meet transplant antigens?. Nature medicine, 8(3), 220-222.
[4] Collins, A. J., Li, S., Gilbertson, D. T., Liu, J., Chen, S., & Herzog, C. A. (2003). Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney International, 64.
[5] Ferguson, R., et al. “Immunosuppression with Belatacept‐Based, Corticosteroid‐Avoiding Regimens in De Novo Kidney Transplant Recipients.” American Journal of Transplantation 11.1 (2011): 66-76.
[6] Gajarski, Robert J., et al. “Infection and malignancy after pediatric heart transplantation: the role of induction therapy.” The Journal of Heart and Lung Transplantation 30.3 (2011): 299-308.
[7] Groth, Carl G., et al. “SIROLIMUS (RAPAMYCIN)-BASED THERAPY IN HUMAN RENAL TRANSPLANTATION: Similar Efficacy and Different Toxicity Compared with Cyclosporine1, 2.” Transplantation 67.7 (1999): 1036-1042.
[8] Gruessner, Rainer WG, et al. “Calcineurin inhibitor-and steroid-free immunosuppression in pancreas-kidney and solitary pancreas transplantation.” Transplantation 79.9 (2005): 1184-1189.Takemoto, S., Port, F. K., Claas, F. H., & Duquesnoy, R. J. (2004).
[9] Halloran, Philip F. “Immunosuppressive drugs for kidney transplantation.” New England Journal of Medicine 351.26 (2004): 2715-2729.
[10] He, J., Li, Y., Zhang, H. (2014). “Immune function assay (ImmuKnow) as a predictor of allograft rejection and infection in kidney transplantation.” Clinical Transplant. (27).
[11] HLA matching for kidney transplantation. Human immunology, 65(12), 1489-1505.
[12] Huh, Kyu Ha, et al. “Exchange living-donor kidney transplantation: merits and limitations.” Transplantation 86.3 (2008): 430-435.
[13] Kahan, Barry D., et al. “IMMUNOSUPPRESSIVE EFFECTS AND SAFETY OF A SIROLIMUS/CYCLOSPORINE COMBINATION REGIMEN FOR RENAL TRANSPLANTATION1.” Transplantation 66.8 (1998): 1040-1046.
[14] Kauffman, H. Myron, et al. “Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies.” Transplantation 80.7 (2005): 883-889.
[15] Levey, A. S. (2003). National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Annals of Internal Medicine Ann Intern Med, 139(2), 137.
[16] Pescovitz, Mark D., and Mahendra Govani. “Sirolimus and mycophenolate mofetil for calcineurin-free immunosuppression in renal transplant recipients.” American journal of kidney diseases 38.4 (2001): S16-S21.
[17] Sandsmark, D. K., Messé, S. R., Zhang, X., Roy, J., Nessel, L., Hamm, L. L., . . . Kasner, S. E. (2015). Proteinuria, but Not eGFR, Predicts Stroke Risk in Chronic Kidney Disease. Stroke, 46(8), 2075-2080.
[18] Schubert, C., van Langeveld, M. C., & Donoso, L. A. (2013). Innovations in 3D printing: a 3D overview from optics to organs. British Journal of Ophthalmology, bjophthalmol-2013.
[19] Sher, J. (2012, January 23). Ontario, B.C. residents wait longer for kidney transplants than any other Canadians. The Star. Retrieved January 26, 2016, from http://www.thestar.com/news/canada/2012/01/23/ontario_bc_residents_wait_longer_for_kidney_transplants_than_any_other_canadians.html
[20] Wood, K. J., & Goto, R. (2012). Mechanisms of rejection: current perspectives. Transplantation, 93(1), 1-10.