Table of Contents
Diabetes
Greek for siphon referring to excessive urination
General Information
Diabetes
Diagnosis : oral glucose tolerance test, Fasting blood glucose test, A1C test
Diabetes is often thought of as one serious illness. However, there are many different types of diabetes that are collectively characterized by one common factor: elevated glucose levels in the blood. The three major types of diabetes include: type 1 or juvenile-onset diabetes, type 2 or adult-onset diabetes, and gestational diabetes. All the different types of diabetes share similar symptoms and complications. Symptoms involve frequent thirst and urination, sudden weight loss and numbness in the limbs. The complications that can develop may involve blindness, organ failure and amputation of the limbs (Bethesda, 2013).
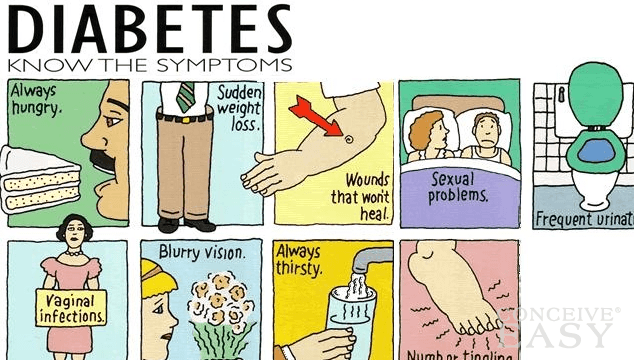 Figure 1: A diagram that depicts the symptoms common to all types of diabetes.
Figure 1: A diagram that depicts the symptoms common to all types of diabetes.
Type I Diabetes
Type 1 diabetes occurs when an individual’s immune system cells attack the body’s own pancreatic cells. Specifically, they attack the beta islet cells, which secrete a hormone called insulin when blood glucose levels are high. Insulin allows glucose to permeate into cells to meet their metabolic demands. The attack of the beta cells by the body results in depleted levels of insulin, resulting in abnormally high levels of glucose in the blood (Stem Cell Network, 2009).
The exact cause of type 1 diabetes remains unknown however researchers speculate a link between environmental stressors and immune system related genes. This means that exercise and a healthy diet can be beneficial to type 1 diabetes patients but will not prevent the disease from developing (Stem Cell Network, 2009).
 Figure 2: A diagram of pancreas producing insulin, which acts to regulate glucose.
Figure 2: A diagram of pancreas producing insulin, which acts to regulate glucose.
Type II Diabetes
Type 2 diabetes mellitus occurs when the beta-cells of the pancreas fail to create sufficient amounts of insulin, or when insulin is not used efficiently by the body (insulin resistance). Type 2 diabetics with insulin resistance can even produce an abnormally high amount of insulin that results in hyperinsulinemia. Muscle cells and other glucose-consuming cells do not respond to the binding of insulin on insulin receptors. The result is a lack of signaling pathways that signal for the translocation of insulin-dependent glucose transporter type 4 (GLUT4) to cell membranes. The absence of GLUT4 transporters means a high level of glucose in the cardiovascular system. The net effect of this pathophysiology is a chronic increase in blood glucose levels.
In addition, when an individual becomes sick that has type 2 diabetes who is not able to drink fluids will result in developing diabetic coma. This condition is life threatening. Individuals that are more likely to get type 2 diabetes are people that are older then the age of 45, overweight and also don’t participate in fitness activities such as physical exercises. It is also known that type 2 diabetes can be inherited from family members but the process involved isn’t understood well. (Brind’Amour, 2014).
Gestational Diabetes
Gestational diabetes is not a permanent condition but it can be harmful to both the mother and the fetus. About 2 to 4% of pregnancies will develop gestational diabetes. (Canadian Diabetes Association, 2014).
The Evolution of Diabetes
The increase in the levels of diabetes is attributed to the widest spreading disease in the world right now western society. Between 1985 and 2002 the number of people with diabetes has grown exponentially from 30 million to 217 million. This number is projected to increase to 366 million by 2030. So evolutionarily speaking why is the number of individuals increasing so rapidly. It has to do with a gene that encodes a protein that helps move lipids into the liver. Carriers of a mutation in this gene are more susceptible to get the disease at an earlier age (Young et al., 2002). However, certain populations of people are more likely to carry a mutation in the gene. Individuals with a Native American background have a greater than 50% chance of carrying at least of of the deformed genes. While in individuals with a European ancestry a carrier is rare. It is hypothesized that the gene in question evolved over 800,000 years ago, when we were mere neanderthals. New fossil records validate this, as the gene has been found in ancient Neanderthals. Never before have we seen such strong natural selection against a gene (Selam, 2010)
Risk Factors
Over 2.5 million Canadians suffer from Diabetes. 60% of individuals who are obese suffer from diabetes. There is a genetic factor for diabetes having a sibling or parent with the disease increases your chances of suffering from the disease. High risk populations are Hispanics, Aboriginals, South Asians, Asians, or individuals from African decent. Having high blood pressure, high cholesterol, and being diagnosed with prediabetes increases your chances of suffering from diabetes.
Reducing Risk Factors
Recent studies done by the American Diabetes Association are showing that high doses of active form of vitamin D3, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) prevent diabetes in the non-obese diabetic (NOD) mouse. However, these high doses are causing unwanted calcemic side effects. Animal studies show that lifelong treatment raised vitamin D levels from 173 nmol/L to 290 nmol/L, in control mice. This is attributed to a suppression in immune function which preserved pancreatic insulin levels. Furthermore, vitamin D3, decreased interferon-γ-positive CD8+ T-cells and increased CD4+(CD25+)FoxP3+ T-cells in pancreatic draining lymph nodes.
Novel Treatments
Since the discovery of insulin in 1921, Diabetic patients have been able to rely on the health care industry to find medication and treatment for the disease. Today, patients have a plethora of treatment options varying from insulin injections, oral medications, stem cell therapy and alternative supplements.
Type 1 Diabetes: Stem Cell Therapy
Previous Treatment Attempts
There is currently not cure for type 1 diabetes. Affected individuals are provided with daily insulin shots and must keep strict supervision of their blood glucose concentrations in order to avoid complications.
In an effort to cure and eradicate type 1 diabetes, pancreatic transplantations were developed and have been performed for several decades. These transplantations were highly successful. In fact, eighty-three percent of individuals that underwent transplants discontinued their daily insulin shots and showed no signs of diabetes for many months following the transplant. However, the benefits were compromised by many complicated, life-threatening side effects. The biggest side effect being that individuals had to become immunosuppressed for the rest of their lives. Immunosuppression renders individuals susceptible to infectious diseases as the immune system is prevented from reacting to foreign particles. Therefore, this makes pancreatic transplantations more dangerous than the diabetes itself (Bethesda, 2013).
Stem Cells: A New Horizon
The idea of transplanting stem cells into type 1 diabetic patients arose in an effort to avoid immunosuppression and organ rejection (Stem Cell Network, 2009). Stem cells are unspecialized cells that can self-renew and may differentiate into any of the body’s cell types. The goal of an effective therapy is to overcome both the deficiency in pancreatic beta cells and the autoimmune attack by the body (Aguayo-Mazzucato & Bonner-Weir, 2010). Consequently, stem cell treatment aims to direct these undifferentiated cells towards becoming unlimited reservoirs of insulin-producing beta cells in the affected individual (Stem Cell Network, 2009).
There are many sources of stem cells that can be handled in different ways to produce beta cells. The main sources involve human embryonic stem cells, induced pluripotent stem cells, fetal stem cells and adult stem cells (Bethesda, 2013).
 Figure 3: Differentiation of stem cells into mature, organ-specific cells.
Figure 3: Differentiation of stem cells into mature, organ-specific cells.
Human Embryonic Stem Cells (hESCs)
hESCs are the most useful stem cells because they are pluripotent. This means that they can differentiate into any cell type that derives from any of the three germ cell layers: endoderm, mesoderm and ectoderm (Stem Cell Network, 2009).
To obtain insulin-producing beta cells from hESCs, a proper endoderm layer must be developed. The endoderm is the innermost layer produced during embryogenesis that differentiates into endocrine glands and organs, the gastrointestinal tract and the respiratory tract (R&D Systems, Inc., 2014). This layer can be established in the hESCs by introducing the essential transcription factors and handling the signaling pathways listed below. In addition to these mandatory components, there are several other transcription factors and growth hormones that aid in beta cell development but are not required for proper differentiation of the cells (Aguayo-Mazzucato & Bonner-Weir, 2010).
Insulin-producing beta cells have been developed from hESCs in vitro by researchers over the last decade. However, there are two main limitations to these beta cells. The first limitation has been a lack of responsiveness to glucose despite their insulin-producing qualities, rendering them useless in regulating elevated glucose levels. Furthermore, the second limitation has been the creation of poly-hormonal beta cells. That is, beta cells that secrete the three main pancreatic hormones: insulin, glucagon and somatostatin (Rezania et al., 2014).
Required Signaling Pathways and Transcription Factors for Beta Cells
The signaling pathways required for development of the endoderm layer in hESCs are:
- TGF-beta Superfamily Signaling Pathway: Establishes endoderm cell precursors with Activin A and Nodal proteins
- Wnt Signaling Pathway: Role in pancreatic development with Activin A
- Hedgehog Signaling Pathway: Inhibits pancreatic development (pathway must be inhibited)
- Notch Signaling Pathway: Inhibits pancreatic development (pathway must be inhibited)
The transcription factors that activate genes for proper beta cell development in hESCs are:
- Pancreas and Duodenum Homeobox Protein 1 (PDX1): For beta cell differentiation
- Neurogenin-3 (NGN3): For insulin production
- Neurogenic Differentiation Factor 1 (NEUROD1): Activation/repression of insulin transcription factors
- Paired Box Protein 4 (PAX4): Beta cell growth
- Transcription Factor MAFA: Insulin production due to glucose
Sources: (Aguayo-Mazzucato & Bonner-Weir, 2010) and (R&D Systems, Inc., 2014)
Induced Pluripotent Stem Cells (iPSCs)
iPSCs are generated when dedifferentiation occurs. In other words, a mature cell that has already undergone differentiation is turned back to its pluripotent state. This pluripotent cell can later be differentiated into another cell type (Aguayo-Mazzucato & Bonner-Weir, 2010).
These newly pluripotent cells are commonly obtained from human somatic cells such as skin cells (Stem Cell Network, 2009). In the past, a combination of transcription factors and oncogenes were used to generate iPSCs from skin cells however, this increased the risk of developing tumors. Using valproic acid, which allowed the oncogenes to be omitted from use, lowered the risk (Aguayo-Mazzucato & Bonner-Weir, 2010).
In order to introduce the transcription factors into the skin cells, retroviruses were used. However this also increased the risk for tumor development and thus, novel therapies were developed without the use of viral vectors (Aguayo-Mazzucato & Bonner-Weir, 2010). These therapies insert the transcription factor genes into plasmids via transfection. These plasmids, which are expression vectors, express the inserted gene when it enters the skin cell (Okita, Nakagawa, Hyenjong, Ichisaka & Yamanaka, 2008). Once the iPSCs are obtained, they can differentiate into endoderm cells by the same process described for hESCs (Maehr et al., 2009).
Fetal Stem Cells
Fetal stem cells and pancreatic precursor cells have been studied however the main source of stem cells may derive from the umbilical cord. This is because it contains stem cells that may differentiate and suppress the autoimmune attacks that occur during type 1 diabetes (Aguayo-Mazzucato & Bonner-Weir, 2010).
Adult Stem Cells
Beta cell precursors can be found in some adult organs such as the pancreas and the liver. These precursor cells are very valuable as they are partly differentiated and are more likely to continue differentiating into islets of Langerhans. Specifically, pancreatic duct epithelial cells, pancreatic acinar cells and liver cells have been found to be potential beta cell precursors (Aguayo-Mazzucato & Bonner-Weir, 2010).
Latest Research: Beta-like Cells Derived from hESCs
A recent study by Rezania et al. has found a way to overcome the two main limitations of beta cell development from hESCs. Researchers were able to derive cells in vitro that secrete only insulin and respond to high glucose levels.
The study used a seven-stage protocol that produced cells with very similar characteristics as beta cells, such as the presence of important transcription factors. Although they were not beta cells, these stage S7 cells seemed to cure type 1 diabetes in mice models about six to ten weeks following transplantation as shown in figure 4. Specifically, figure 4b shows the insulin production of S7 cells in diabetic mice during several time periods while figure 4d shows the same information for non-diabetic mice used as controls.
These results are significant as diabetic symptoms are eliminated in an animal model about four times faster than in any previous study. This may lead researchers towards a better treatment for type 1 diabetes and potentially, a cure (Rezania et al., 2014).
Type 2 Diabetes: DPP-4 Inhibitors & Combination Drugs
The first dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, under the trade name Januvia, was approved by the FDA in 2006. Since then, there have been major developments in the varieties of DPP-4 inhibitors used to combat type 2 diabetes. As of 2013, there are 8 types of DPP-4 inhibitors worldwide, including sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin, and more recently, gemigliptin, anagliptin and teneligliptin (Deacon & Holst, 2013). These DPP-4 inhibitors serve to protect glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) from being degraded by the enzyme DPP-4 (Deacon & Holst, 2013). These inhibitors are administered with other therapies when desired therapeutic effects are not seen in patients using only metformin. DPP-4 inhibitors can also be administered as a mono-therapy if there are no other options available (Rodbard et al., 2009).
DPP-4 inhibitors are easily administered, has good tolerance, and has a low risk for hypoglycemia. Additionally, it does not alter patient body weight, and has rare incidences of adverse effects (Monami et al., 2011). Thus, these inhibitors are favourable for the vulnerable portion of the population, specifically for elderly people.
Classic Mechanism of Action
GLP-1 and GIP are endogenous, insulin-dependent incretin hormones secreted by the gut in response to high levels of glucose, and serve to lower blood glucose by enhancing insulin release from the pancreas. Specifically, GLP-1 inhibits glucagon secretion, delays gastric emptying, and suppresses appetite, and thus, reduces body weight (Deacon & Holst, 2013). Additionally, both GLP-1 and GIP enhances all steps in insulin biosynthesis, as well as improve beta-cell response to the presence of glucose (Deacon & Holst, 2013). Incretin hormones GLP-1 and GIP are substrates for the enzyme DPP-4, whose function is to cleave active forms GLP-1(7-36) and GIP(1-42) to inactive forms GLP-1(9-36) and GIP(3-42), respectively (Zhong et al., 2012). The DPP-4 enzyme is expressed throughout the body. The enzyme DPP-4 was targeted in finding treatments for type 2 diabetes, due to its ability to degrade incretins GLP-1 and GIP.
DPP-4 inhibitors are a heterogeneous class of small molecules with different chemical structures, metabolism, and elimination. A variety of inhibitors have similar efficacies in terms of reducing blood glucose levels and glycated hemoglobin (HbA1c) levels (Deacon & Holst, 2013). Since the chemical structures of these inhibitors vary, each of their binding modes in DPP-4 would be unique. In general, DPP-4 inhibitors bind reversibly to DPP-4 by forming H-bonds and by participating in hydrophobic interactions within the DPP-4 enzyme subsites (Nabeno et al., 2013). The binding of the inhibitor to the DPP-4 enzyme renders the enzyme inactive, therefore allowing GLP-1 and GIP to remain in circulation for a longer period of time.
The classical mechanism of action for DPP-4 inhibitors is the inhibition of DPP-4 in blood circulation, and thus prevent its action in degrading GLP-1 and GIP (Omar & Ahren, 2014). The net result is a higher concentration of active, circulating incretin hormones and thus, enhances insulin release and inhibition of glucagon release. These actions ultimately aid in lowering blood glucose and glycated hemoglobin A1c levels (Omar & Ahren, 2014). All of the DPP-4 inhibitors used in clinical settings have long-lasting inhibition effects, and thus dosing schedules do not need to be frequent. For example, sitagliptin can inhibit the action of DPP-4 for a 24 hour period (Omar & Ahren, 2014).
Pleotropic Mechanism of Action
In addition to the classical mechanism of action of DPP-4 inhibitors, there are several other non-classical mechanisms of action of DPP-4 inhibitors that may contribute to the glucose-lowering effect of these inhibitors:
- Inhibition of gut DPP-4 activity
- Inhibition of islet DPP-4 activity
- Inhibition of other active peptides other than GLP-1
Source: (Omar & Ahren, 2014)
The mechanisms mentioned above are are only proposed mechanisms. More research needs to be conducted to approve or disprove these propositions.
Incretin Mimetics (GLP analogs)
These types of drugs include the injected drugs Byetta, Tanzeum, and Victoza. They use the body's own signaling system to boost insulin after meals.
Symlin
Pramlintide, under the trade name Symlin, is an injectable synthetic hormone and analog of human amylin. It is used in conjunction with insulin to help lower blood sugar after meals in type 1 or 2 diabetic patients who fail to achieve the desired glucose level despite insulin therapy. It complements insulin by slowing gastric emptying, inhibiting post-meal glucagon, and decreasing appetite. The net effect is a decrease in food intake, and a lowering of body weight and overall better glucose control in comparison to insulin alone (Herrmann et al., 2014). However, side effects often include nausea, and hypoglycaemia in rare cases. Overall, Symlin is safe for diabetic patients with varying amounts of insulin.
Combination Drugs
Combination drugs have made a huge difference in the treatment of diabetes mellitus. These drugs are made with different medications combined in one pill. The combination often consists of metformin and a sulfonylurea, a meglitinide, a DPP-4 inhibitor, a thiazolidinedione, or a thiazolidinedione (Joslin Diabetes Center, 2014). This is beneficial for the patient, in that this allows a reduction in the number of pills that needs to be ingested. Combination drugs include Actoplus MET, Avandamet, Duetact, Glucovance, Metaglip, Kazano, Oseni, and PrandiMet (Joslin Diabetes Center, 2014). The side effects of these drugs are the same as the side effects exhibited if the individual drugs were taken separately. Drawbacks of taking the aforementioned drugs include a higher cost; it is generally more expensive than the individual drugs combined.
Type 2 Diabetes: Metformin
Introduction
Also known as: Glucophage; Glumetza; 1,1-Dimethylbiguanide; Metiguanide; Diabex; Dimethylbiguanide; Metforminum; Diabetosan; Fluamine Metformin, most commonly sold under the name of Glucophage is the most prescribed oral anti-diabetic drug with over 120 million users worldwide. Metformin was originally obtained by extraction from the Galega officinalis (French iliac) in the 1920s and had its beginnings as a large-scale drug in 1957 in Europe and 1995 in the United States (PubMed, 2014). Initially known for it's effectiveness at being an anti-hyperglycemic for type II diabetes, metformin is now also popular for treating polycystic ovary syndrome and being explored for it's possible use in cancer treatment (Pernicova & Korbonits, 2014)
Chemical Structure and Properties
Figure 5: Chemical structure of metformin.
IUPAC Name: 3-(diaminomethylidene)-1,1-dimethylguanidine
Molecular Formula: C4H11N5
Class: Biguanide
At physiological conditions, metformin's amino groups will act as bases and attack hydrogen protons giving metformin a positive charge. Metformin's charge and high polarity make it necessary for the drug to use cell membrane transporters for it's uptake and secretion (ie SLC22A1). These transporters are found in high concentration in liver cells and in lower concentrations in skeletal muscle cells (Pernicova & Korbonits, 2014)
Metformin & Type II Diabetes
In general, metformin is used extensively to treat type II diabetes because of it's glucose-lowering action. Despite the drug's popularity, the mechanism by which it lowers blood glucose it yet to be well defined. Multiple metabolic processes are affected with the overall result being an increase in the amount of glucose entering hepatic and skeletal muscle cells and a reduction in the amount of glucose leaving hepatic cells. Cellular metabolic processes such as glucose transport, glycolysis, gluconeogenesis, and glycogen synthesis are all known to be altered by metformin and contribute to the overall decrease in blood glucose concentration (Pernicova & Korbonits, 2014)
 Figure 6: For diabetes mellitus; metformin lowers glucose levels and improves insulin sensitivity.
Figure 6: For diabetes mellitus; metformin lowers glucose levels and improves insulin sensitivity.
Metformin: Mechanisms of Action
Increased Glucose Uptake: Increased insulin receptor activity and expression and increased GLUT-1 (SLC22A-1) and GLUT-2 (SLC22A-4) translocation increases the amount of glucose entering hepatic and skeletal muscle cells from the cardiovascular system (Pernicova & Korbonits, 2014)
Decreased Gluconeogenesis: Gluconeogenesis: metabolic process of glucose production from non-carbohydrate sources such as lactate and certain amino acids Gluconeogenesis is normally increased by 25%-98% in type II diabetic patients but metformin will lower amount of gluconeogenesis by 75%. When metformin enters the cell, research leads us to believe that it alters complex 1 of the hepatic cell's mitochondria. As a result, the electron transport chain (ETC) is inhibited therefore causing ATP production to be greatly reduced and the cell's ATP:AMP ratio to also fall. The rising AMP will cause cAMP production to decrease and for 5-AMPK to be activated. cAMP is associated with glucagon signalling and switching on gluconeogenesis and 5-AMPK activation is responsible for turning on glycolysis, inhibiting gluconeogenesis, increasing insulin receptor activity, increasing GLUT-1 translocation, and decreasing fatty-acid deposition. By preventing fatty acids from accumulating in cells it will improve insulin sensitivity in the liver. The main overall results, is a dramatic reduction in gluconeogenesis, an insensitivity to glucagon, and decreased fat composition in the liver (Pernicova & Korbonits, 2014)
REDUCTION IN THE INCIDENCE OF TYPE 2 DIABETES WITH LIFESTYLE INTERVENTION OR METFORMIN
Main goal of the paper was to reduce the occurrence of diabetes by the use of metformin or lifestyle changes. Arbitrarily, 3234 individuals were picked from which one group was given placebo, second group consumed 850 mg of metformin twice throughout the day, and the last group involved changes in the lifestyle.After about 2.8 years it was shown statistically that the lifestyle change did in fact reduce the occurrence of diabetes by 58 percent. Surprisingly, the intake of metformin reduced it by 31 percent. This indicates that consumption of metformin is effective towards reducing the occurrence of diabetes (Pernicova & Korbonits, 2014).
METABOLIC EFFECTS OF METFORMIN IN NON-INSULIN-DEPENDENT DIABETES MELLITUS
Overall, the article indicates that glycosylated hemoglobin value decreased, also lowered the plasma glucose concentrations. In addition, the patients lost about 88 percent of adipose tissue.Ultimately, metformin works by reducing output of hepatic glucose output. As a result, it constrains gluconeogenesis(Pernicova & Korbonits, 2014).
METFORMIN: A DIABETES DRUG FOR CANCER, OR A CANCER DRUG FOR DIABETICS?
Evidence portrays that individuals with diabetes that receive metformin have a decreased occurrence of cancer.Basically, metformin affects the proliferation of the cells related to the tumor. It inhibits the first complex of the mitochondrial electron transport chain. Ultimately, blocking oxidative respiration. Overall, metformin acts as a barrier towards the cancer by constraining the Raptor/mTOR (mTORCI) complex (Pernicova & Korbonits, 2014).
References
Aguayo-Mazzucato, C., & Bonner-Weir, S. (2010). Stem cell therapy for type 1 diabetes mellitus. Nature Reviews Endocrinology, 6(3), 139-148.
Bethesda, MD: National Institutes of Health, US. (2013). Stem Cells and Diabetes. In Stem Cell Information (World Wide Web site). Retrieved from http://stemcells.nih.gov/info/scireport/pages/chapter7.aspx.
Brind’Amour, K. (2014). What do You Want to Know About Type 2 Diabetes? Retrieved from: http://www.healthline.com/health/type-2-diabetes
Canadian Diabetes Association. (2014). Types of Diabetes. In Canadian Diabetes Association. Retrieved from http://www.diabetes.ca/about-diabetes/what-is-diabetes.
Deacon, C.F. & Holst, J.J. (2013). Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin. Pharmacother., 14(15), 2047-2057.
Herrmann, K., Shan, K., Brunell, S.C., & Chen, S. (2014). Effects of pramlintide in patients with type 2 diabetes mellitus: An analysis using daily insulin dose tertiles. American Association of Clinical Endocrinologists, 20(9), 1-21.
Knowler, W., Connor, E., Fowler, S., Hamman, R., Lachin, J., Walker, E., & Nathan, D. (2002, February). Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metaformin. N Engl J Med, 346(6), 393-403.
Monami, M., Dicembrini, I., Martelli, D., & Mannucci, E. (2011). Safety of dipeptidyl peptidase-4 inhibitors: A meta-analysis of randomized clinical trials. Current Medical Research and Opinion, 27(3), 57-64.
Joslin Diabetes Center. (2014). Oral Diabetes Medications Summary Chart. Retrieved from http://www.joslin.org/info/oral_diabetes_medications_summary_chart.html.
Liu, Y., & Hong, T. (2014). Combination therapy of dipeptidyl peptidase-4 inhibitors and metformin in type 2 diabetes: rationale and evidence. Diabetes Obes. Metab., 16, 111-117.
Maehr, R., Chen, S., Snitow, M., Ludwig, T., Yagasaki, L., Goland, R., … & Melton, D. A. (2009). Generation of pluripotent stem cells from patients with type 1 diabetes. Proceedings of the National Academy of Sciences, 106(37), 15768-15773.
Nabeno, M., Akahoshi, F., Kishida, H., Miyaguchi, I., Tanaka, Y., Ishii, S., & Kadowaki, T. (2013). A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochemical and Biophysical Research Communications, 434(2), 191-196.
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T., & Yamanaka, S. (2008). Generation of mouse induced pluripotent stem cells without viral vectors. Science, 322(5903), 949-953.
Omar, B. & Ahrén, B. (2014). Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes, 63, 2196-2202.
Pernicova, I., Korbonits, M. (2014). Metformin-mode of action and clinical implications for diabetes and cancer. Nature Reviews Endocrinology, 1-14.
Rezania, A., Bruin, J. E., Arora, P., Rubin, A., Batushansky, I., Asadi, A., … & Kieffer, T. J. (2014). Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology.
Rodbard, H.W., Jellinger, P.S., Davidson, J.A., Einhorn, D., Garber, A.J., Grunberger, G., … & Schwartz, S.S. (2009). Statement by an American Association of Clinical Endocrinologists/ American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocrine Practice, 15(6), 540-59.
R&D Systems, Inc. (2014). Pancreatic Endoderm. In R&D Systems A Bio-techne Brand. Retrieved from http://www.rndsystems.com/molecule_group.aspx?g=801.
Pernicova, I., & Korbonits, M. (2014, January). Metformin- Mode of Action and Clincial Implications for Diabetes and Cancer. Nature Reviews, 10(2), 1-14.
Selam, J. L. (2010). Evolution of diabetes insulin delivery devices. Journal of diabetes science and technology, 4(3), 505-513.
Stem Cell Network. (2009). Type 1 Diabetes. In Stem Cell Network. Retrieved from http://www.stemcellnetwork.ca/index.php?page=type-1-diabetes.
Stumvoli, M., Nurjhan, N., Perriello, G., Dailey, G., & Gerich, J. (1995, August). Metabolic Effects of Metformin in Non-Insulin-Dependent Diabetes Mellitus. The New England Journal of Medicine, 333(9), 550-554.
Young, R. J., Khong, C. K., Vaughan, N. J. A., New, J., & Roxburgh, M. (2002). The evolution of diabetes information systems. Diabetic Medicine, 19(s4), 6-12.
Zhong, J., Rao, X., & Rajagopalan, S. (2012). An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis.

