Table of Contents
Aplastic Anemia
Introduction
Aplastic Anemia is a rare blood disease that causes bone marrow to limit its production of red blood cells, white blood cells and blood platelets. Bone marrow is responsible for the making of stem cells, which then differentiate into more specific cells like red blood cells. Red blood cells are important for the circulation of oxygen throughout the body and the removal carbon dioxide, white blood cells play an important role in the immune system and platelets help clot blood. A limited amount of these cells could mean problems in energy levels, increased susceptibility to infection and excess blood loss (NIH, 2012).
Origin & Background
Hematology the word can be broken down into two parts hemat- (meaning blood) and –ology (meaning the study of). There are a wide variety of diseases associated with the blood including aplastic anemia, polycythemia, myelofibrosis, leukemia, haemophilia and more (Mandal, 2013).
Common Types of Anemia
- Iron deficiency anemia: is the most common of the seven disorders, it involves extra loss of blood through menstruation, making the body want more iron.
- Thalassaemia: acquired from parents causing the body to generate less red blood cells and haemoglobin.
- Aplastic Anemia: bone marrow creates less red blood cells, white blood cells and blood platelets.
- Haemolytic anemia: red blood cells are targeted and killed prematurely by the body.
- Sickle cell anemia: abnormally shaped blood cells that are sickle shaped that clump and block blood vessels.
- Pernicious anemia: vitamin B12 deficiency causing generation of unhealthy red blood cells.
- Fanconi anemia: a more specific type of aplastic anemia disorder leading to defective bone marrow (Health 24, n.d.).
Discovery
 There is a bit of discrepancy between who actually discovered aplastic anemia. Dr. Paul Ehrlich first treated the disease in 1888. He was observing a young woman who died prematurely from an illness. Her symptoms were limited amounts of red blood cells, excessive bleeding and fevers, similar to what we would now expect as aplastic anemia. Dr. Anatole Chauffard officially introduced the name “Aplastic Anemia” years later in 1904. Originally it was thought that bone marrow only produced red blood cells, but it wasn’t until later that it was discovered that white blood cells and platelets were also limited in the body (Cuglievan, DePombo, & De Angulo , 2017).
Due to the complexity of the disease and its involvement in not one, but three types of cells, its not surprising it has been hard to find a cure that covers the entirety of the disease.
There is a bit of discrepancy between who actually discovered aplastic anemia. Dr. Paul Ehrlich first treated the disease in 1888. He was observing a young woman who died prematurely from an illness. Her symptoms were limited amounts of red blood cells, excessive bleeding and fevers, similar to what we would now expect as aplastic anemia. Dr. Anatole Chauffard officially introduced the name “Aplastic Anemia” years later in 1904. Originally it was thought that bone marrow only produced red blood cells, but it wasn’t until later that it was discovered that white blood cells and platelets were also limited in the body (Cuglievan, DePombo, & De Angulo , 2017).
Due to the complexity of the disease and its involvement in not one, but three types of cells, its not surprising it has been hard to find a cure that covers the entirety of the disease.
Etiology
Aplastic Anemia is caused either as a result of the accidental impairment of stem cells by the immmune system or because of genetic-related factors (AAMDS, 2016).
Acquired Aplastic Anemia
Acquired Aplastic anemia is characertized as an autoimmune disease and can develop at any point in one's life (NIH, 2012). It is linked to exposure of toxins(such as pesticides, arsenic, or benzene), radiation/chemotherapy used for treating cancer/disorders, medications used for the treating autoimmune diseases like rheumatoid arthritis and infectious diseases such as Hepatitis and HIV (NIH, 2012). Acquired aplastic anemia can also develop through pregnancy but this can be temporary until delivery.
Hereditary Aplastic Anemia
Hereditary Aplastic Anemia is far less common compared to acquired aplastic anemia (AAMDS, 2016). It is passed down from parent to child and is diagnosed during childhood. Fanconi anemia, Shwachman-Diamond syndrome, Dyskeratosis and Diamond-Blackfan anemia are inherited conditions that can harm stem cells contributing to aplastic anemia (AAMDS, 2016).
Recent findings have identified a new type of hereditary aplastic anemia diagnosed in adulthood. It is prevalent in individuals who have family members with a history of aplastic anemia or fibrosis of the lungs or liver (AAMDS, 2016). It is characterized by excessive shortening of the telomeres and is only diagnosed through special tests.
In most cases, the cause of aplastic anemia is unknown and is referred to as idiopathic aplastic anemia (AAMDS, 2016).
Genetic Research
PRF1 Genetic Alterations Research by Solomou et al., suggest that genetic alterations to the Perforin-1 (PRF1) gene contributes bone marrow failure. In the study, researchers obtained DNA from 75 unrelated patients and analysed the DNA sequence for PRF1 (Solomou et al., 2007) . 4/5 patients with the PRF1 mutation diagnosed with aplastic anemia had hemphapgocytosis in the bone marrow (Solomou et al., 2007). This suggests that PRF1 mutation is involved with impaired cytotoxic T-cell activity, low Perforin protein level and a risk factor for bone marrow failure (Solomou et al., 2007).
ACD Genetic Alterations The ACD gene interacts and changes the telomere binding protein (TPP1). Once ACD interacts with telomere and telomerase on the end of the chromosomes, it disrupts the interaction and results in alteration to the telomere function (Guo et al., 2014). ACD gene prevents the telomerase maintaining the telomere and protecting the cells, the blood cell loses structural integrity (Guo et al., 2014). Eventually, the blood cells will die and resulting in bone marrow failure and aplastic anemia.
Epidemiology
Aplastic anemia commonly affects older demographics, particular individuals over the age of 60 years (Young & Kaufman, 2008). However, it can also affect young adults between the ages of 20 to 25 and is equally present in males and females. There is considerable geographic variability associated with aplastic anemia since there is 2-3 fold increase of the disease in East and South-East Asia compared to Europe and North America (Young & Kaufman, 2008). In East Asia working men are disproportionately diagnosed with the disease, likely a result of exposure to toxic substances in the workplace.
A study by Montane and et al., conducted between 1983-2003 reported the incidence rates of aplastic anemia. A total of 235 cases were identified with 2.34 million people diagnosed yearly with the disease. Most cases reported were severe or very severe aplastic anemia, with occurrences increasing with age.
Survival Rate
Montane and et al., reported the survival rate of aplastic anemia after diagnosis based on three selected time frames.
- After 3 months=73%
- After 2 years= 57%
- After 15 years=51%
Older people diagnosed with a more severe form of the disease had a lower survival rate (Montane et al., 2008). There was a two-year survival rate among patients who were treated with bone marrow transplantation. In 49 of the 235 cases identified, individuals were diagnosed because of exposure to drugs, while 21 cases were a result of exposure to toxic agents (Montane et al., 2008).
Symptoms & Clinical Presentation
There are many symptoms associated with aplastic anemia, and a patient may present with any combination of the following
- Fatigue
- Shortness of breath with exertion
- Rapid or irregular heart rate
- Pale skin
- Frequent or prolonged infections
- Unexplained or easy bruising
- Nosebleeds and bleeding gums or prolonged bleeding from cuts
- Skin rash
- Dizziness or headaches
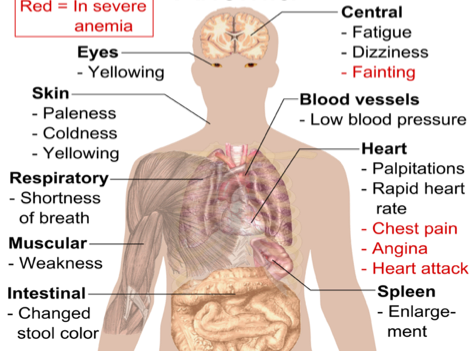
These symptoms may progress slowly over weeks or months, or may present very suddenly in severe cases (Hussain, 2017).
Figure 1: Various symptoms that are observed with aplastic anemia and clinical presentation (Hussain, S. K, 2017).
Myelodysplastic Syndrome
Often aplastic anemia may appear similar to the clinical presentation of myelodysplastic syndrome. It is important to be aware of this syndrome, and look closely for signs that can distinguish the two disorders.
Myelodysplastic Syndrome occurs when the bone marrow is still able to produce new blood cells, but they are deformed or underdeveloped (Myelodysplastic Syndrome, 2017). In some cases this is referred to as hyperplastic bone marrow. Normally, individuals with this syndrome will present with the same symptoms as aplastic anemia, however upon closer examination, their bone marrow will be packed with red blood cells.
Figure 2: The image above depicts abnormally formed blood cells within the bone marrow of a patient with myelodysplastic syndrome (Myelodysplastic Syndrome, 2017).
Pathophysiology
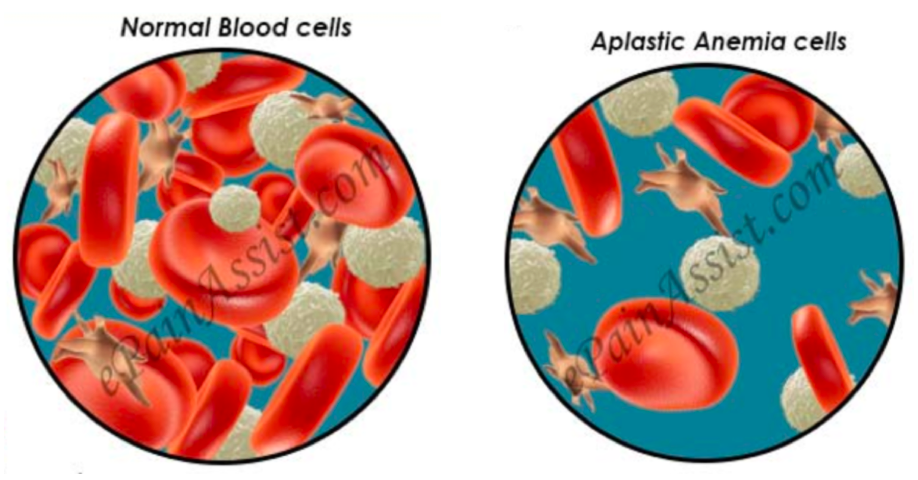
Anemia occurs when the number of red blood cells in the bone marrow is less than normal. Aplastic anemia occurs when the normal production of all blood cells slows or stops completely. This abnormality usually develops when damage has occurred to the bone marrow overtime, slowly shutting down the erythropoiesis, the production of new blood cells.
Figure 3: This image shows the decrease in available blood cells circulating in an individual with aplastic anemia. It is important to note that the blood cells released into the stream are physiologically normal. The decreased number of blood cells is what causes the symptoms of aplastic anemia (Aplastic Anemia & Myelodysplastic Syndromes, 2014).
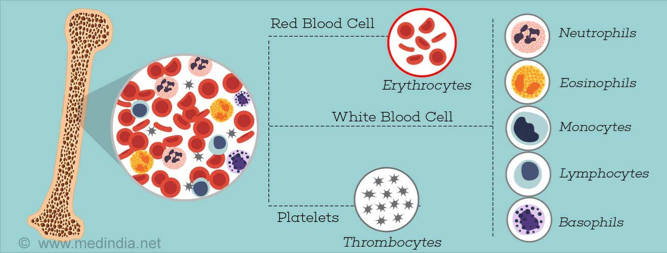
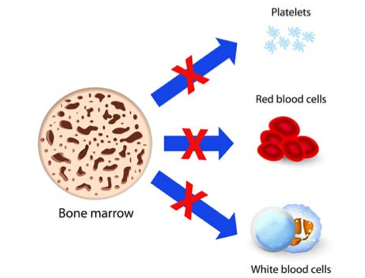
Bone marrow is red and spongy material in your bone that produces stem cells which give rise to a number of cells in the blood (N., 2016). Aplastic bone marrow means the marrow is empty, or contains very few blood cells (hypoplastic). Stem cells in bone marrow produce red blood cells (RBCs), white blood cells (WBCs), and platelets. There are a number of factors that can temporarily or permanently affect the ability of bone marrow to produce blood cells, including: radiation & chemotherapy, exposure to toxic chemicals, use of certain drugs, autoimmune disorders, viral infections, and even pregnancy (N., 2016). When stem cells are damaged from these factors, they are unable to produce blood cells, and further replicate themselves, therefore cell numbers will continue to decrease, limiting the number of blood cells that can be released into the bloodstream.
Figure 4: This image shows the three damaged pathways of the bone marrow stem cells to produce RBCs, WBCs & platelets (Getting to know Aplastic Anemia, 2016).
Stem Cells
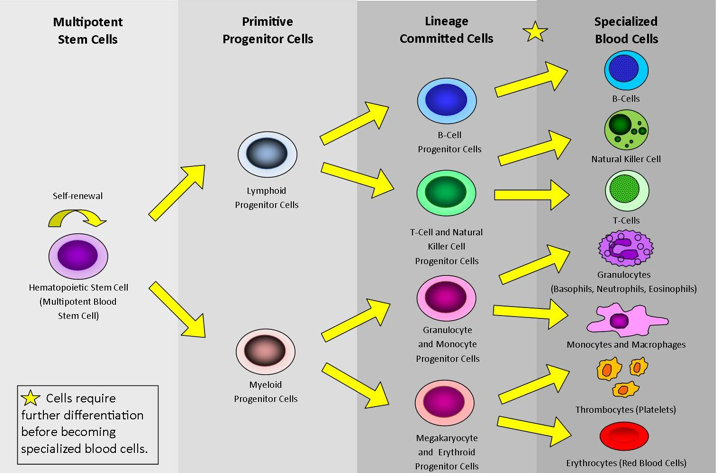
Ten million cells die in your body every minute of every day (Stemple, 2009). Your own stem cells will replace these cells so that you can continue carrying out biological processes you need to stay alive. If stem cells become damaged, you are no longer able to produce blood cells (Stemple, 2009). If this occurs for a long period of time, then the results can be fatal. Stem cells begin as multipotent, where they can differentiate into any cell in the body. Through the process of maturation, they become more specialized as a blood cell, and then further differentiate until full development is reached as a specific blood cell type (Stemple, 2009).
Figure 5: This image depicts the pathway of a multipotent stem cell differentiating into many different specialized blood cells (Stem Cells and Types of Stem Cells, 2017).
Red Blood Cells
Red blood cells typically live in the blood for 120 days, acting as transporters of oxygen and carbon dioxide throughout the circulatory system. Each red blood cell contains an iron-rich hemoglobin protein that carries oxygen to the lungs, and gives blood the characteristic red colour (Skalak et al., 1973). These blood cells make up 40-45% of your blood, making them the second largest blood component next to plasma.
In aplastic anemia, a shortage of functional red blood cells will result in decreased oxygen reaching the lungs and supplying the tissues. Decreased oxygen availability results in shortness of breath and extreme fatigue (Skalak et al., 1973).
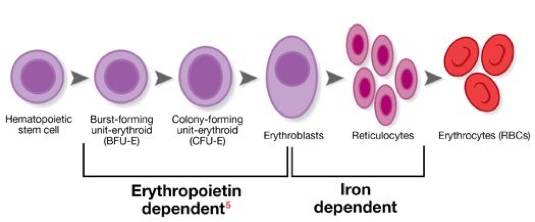
Decreased red blood cells will also reduce the amount of iron circulating in throughout the blood. Decreased iron may cause symptoms of tingling or crawling feeling in the legs, tongue swelling/soreness, pale skin and gums, dizziness, and may also cause an increased or irregular heartbeat (Skalak et al., 1973). Erythropoietin, the formation of new red blood cells, is also an iron dependent pathway required to regenerate new cells every 120 days (Stem Cells and Types, n.d.).
Figure 6: This image shows the development of red blood cells originating as stem cells in the bone marrow to full development as mature red blood cells released into the bloodstream (Stem Cells and Types of Stem Cells, n.d.).
White Blood Cells
White blood cells typically live in the blood for less than a day. These cells are a family of many different types of cells which mediate functions related to immune defense, clossing, bacteria & virus destruction and scavenging of cellular debris (Skalak et al., 1973).
In aplastic anemia, a decrease in white blood cells in the bloodstream will make the body more susceptible to viral and bacteria infections as a result of a reduced immune system defense team (Bacigalupo, 2008).
Platelets
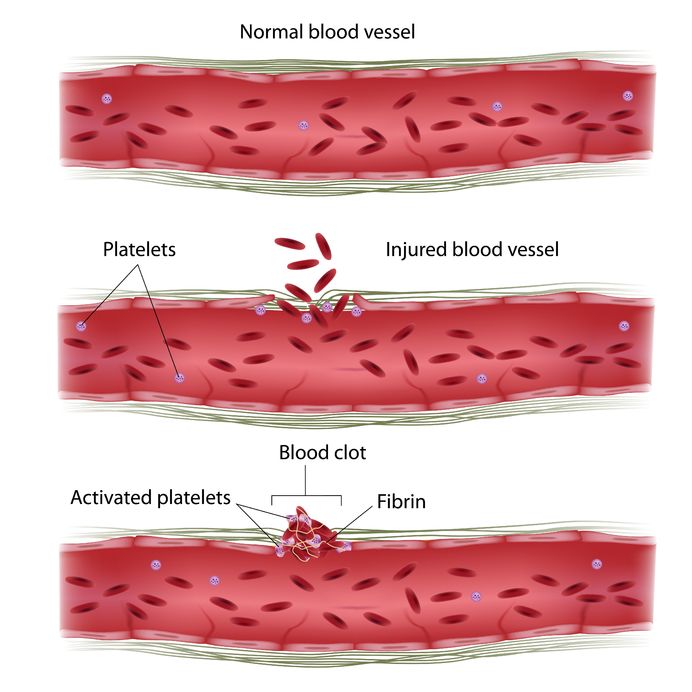
Platelets, also known as thrombocytes, typically live in the blood for around 6 days. These cells function with coagulation factors in the blood such as fibrin to stop bleeding by clumping and creating clots in blood vessel injuries (Bacigalupo, 2008).
In aplastic anemia, a decrease in platelets in the bloodstream will result in bleeding for extended periods of time after an injury occurs. Patients may also present with spontaneous or uncontrolled bleeding as a result of an impaired clotting mechanism (Bacigalupo 2008).
Figure 7: This image shows the role of platelets in the blood stream, working with coagulation factors to clot injured blood vessels (What is a platelet?, n.d.)
Screening & Diagnosis
Aplastic anemia is characterized by reduction in hematopoiesis due to bone marrow damage, therefore diagnostic tests that detect abnormal blood levels and bone marrow damage are administered. These tests include a complete blood count, leukocyte differential, reticulocyte count and bone marrow aspirate and biopsy (NIH, 2017). A complete blood count is administered to evaluate overall health and measure levels of red blood cells, white blood cells, hemoglobin, hematocrit and platelets (NIH, 2017). A leukocyte differential test is administered to measure proportion of white blood cells to each other and detect presence of immature white blood cells (NIH, 2017). A reticulocyte count is administered to measure the speed at which reticulocytes, immature red blood cells, are made by the bone marrow and released into the blood stream (NIH, 2017). In order to measure bone marrow damage by determining bone marrow cellularity, bone marrow aspiration and biopsy are performed (NIH, 2017). During a bone marrow aspiration procedure, a needle is inserted into the bone with a tube attached to it, which creates suction and allows a small sample of bone marrow fluid to be extracted through the tube (NIH, 2017). As well, bone marrow biopsy is performed through insertion of biopsy needle into the bone (NIH, 2017). In this process, the centre of the needle is removed and the hollowed needle is moved deeper into the bone capturing a core of bone marrow within the needle (NIH, 2017). The extracted bone marrow sample undergoes further testing. Diagnosis of aplastic anemia requires performance of bone marrow aspiration and biopsy (NIH, 2017). Severe aplastic anemia is defined by the presence of 2 or more of the following: bone marrow cellularity (hematopoietic stem cell to adipocyte ratio) of 30% or less, absolute neutrophil count of 500 cells per microliter or less, absolute reticulocyte count of 20,000 cells per microliter or less and platelet count of 20,000 cells per microliter or less (Baunstein, 2017). 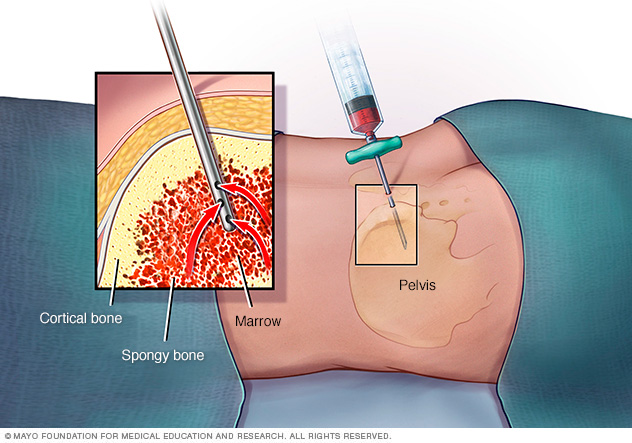 Figure 8: The image displays bone marrow aspiration procedure.
Figure 8: The image displays bone marrow aspiration procedure.
Types
There are three types of Acquired Aplastic Anemia (AAMDS, 2016).
Moderate Aplastic Anemia (MAA) Is the lowest level of severity characterized by low blood cells counts, but not as low compared to severe aplastic anemia (AAMDS, 2016). There are few to no symptoms associated with it and the doctor may not recommend treatment for the patient. However, the doctor may keep an eye on the patient’s blood count. The condition for MAA may stay the same for many years (AAMDS, 2016).
Severe Aplastic Anemia (SAA) Is present when a person exhibits at least two of the following: 1) neutrophil count is less 500 cells per microliter, 2) reticulocyte (young red blood cell) count is less 20,000 per microliter or/and 3) platelet count is less than 20,000 per microliter (AAMDS, 2016).
Very Severe Aplastic Anemia (VSAA) It the most severe form of aplastic anemia. The neutrophil count is less than 200 per microliter and blood count levels are similar those of someone with severe aplastic anemia (AAMDS, 2016).
Treatment & Pharmacology
Immediate Treatment: Blood Transfusions
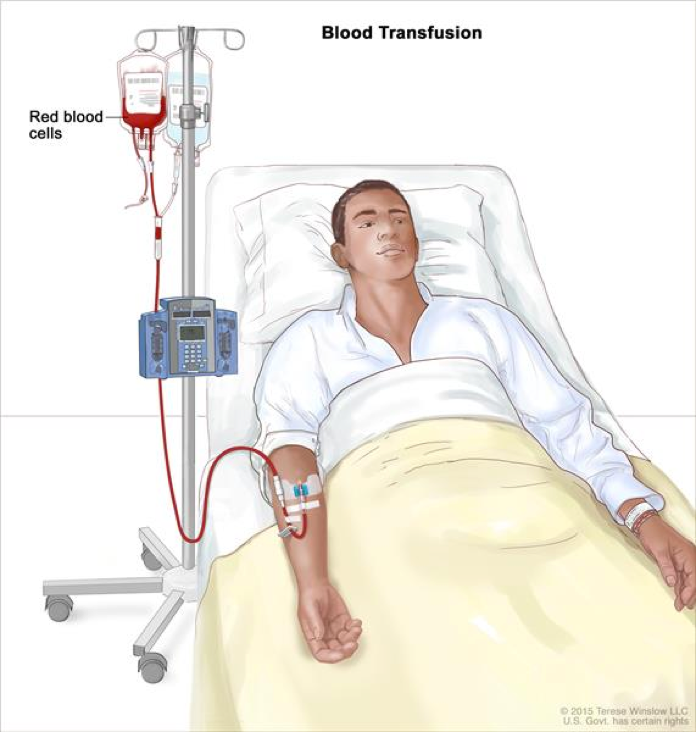
Transfusion therapy readily relieves symptoms of anemia, however is not curative. Blood transfusion is a common procedure in which blood is given through an intravenous line in the patient’s blood vessels. In order to complete a blood transfusion, donor’s blood must be matched with the patient’s blood and filtered to prevent transfusion-associated graft-versus-host disease (Laundy et al 2004; Killick et al, 1997), alloimmunization and reduce incidence of viral infections (Marsh et al, 2010). Blood transfusions from family members should be avoided to prevent sensitization to potential bone marrow donors (DeZern and Brodsky, 2011). The initial aim of transfusion therapy is to correct or prevent cardiopulmonary complication (DeZern and Brodsky, 2011). The goal of platelet transfusion is to maintain high enough platelet count to prevent spontaneous bleeding. Platelet transfusion is suggested when platelet count is below 10,000 µ/l (DeZern and Brodsky, 2011).
Definitive Treatment: Bone Marrow Transplant
Bone marrow transplant is a curative treatment of choice for patients under the age of 30 years who have an HLA-matched sibling donor (DeZern and Brodsky, 2011). The advantage of bone marrow transplant compared to transfusion therapy is reduction in risk of relapse and evolution of myelodysplastic syndrome and paroxysmal nocturnal hemoglobinuria late disorders (Socié et al, 1993). During a bone marrow transplantation, donor stem cells are administered through an intravenous line in the patient’s chest (DeZern and Brodsky, 2011). Transfused stem cells travel to the site of bone marrow and begin producing new blood cells. After procedure, the patient might be offered medication to prevent rejection of donated stem cells as rejection of stem cell transplant may lead to life-threatening complications (DeZern and Brodsky, 2011).
Figure 9: Displayed is the patient undergoing blood transfusion therapy.
Medicines
There are three types of medicines that may be prescribed to aplastic anemia patients such as immunosuppressants, bone marrow stimulants and antibiotics and antiviral medicines (NIH, 2017). Immunosuppressant medicines include cyclosporine anti-thymocyte globulin and methylprednisolone (NIH, 2017). The mechanism of these drugs is to supress the activity of the immune cells that are damaging the bone marrow and consequently to allow for bone marrow recovery and generation of new blood cells. The three immunosuppressants, cyclosporine, anti-thymocyte globulin and methylprednisolone, are often prescribed in combination (NIH, 2017). Immunosuppressants increase the risk of developing leukemia and myelodysplasia, therefore should be prescribed with caution (NIH, 2017). The bone marrow stimulants are man-made substitutes of naturally occurring substances, such as erythropoietin and colony-stimulating factors, that stimulate blood cell production (NIH, 2017). The most frequently prescribed bone marrow stimulants are sagramostim, filgrastim and pegfilgrastim (NIH, 2017). As aplastic anemia patients are susceptible to infections, antibiotics (i.e. ciprofloxacin, neomycin and colistin) and antiviral medicines (i.e. acyclovir and valaciclovir) are also often prescribed (NIH, 2017).
Current Research
Telomerase Based Treatment
One new type of research being implemented for Aplastic Anemia is called Telomerase based treatment. The purpose of the treatment is to introduce telomerase, through gene therapy, into the bone marrow, in hopes that the enzymes will repair telomere length (Bar et al., 2016). Once telomerase is repaired then it will continue to generate blood cells. In the study, there was two strains of mice stimulated acquired Aplastic Anemia while the second strain had a mutation that associated with hereditary aplastic anemia. The results revealed that when both condition was treated with the telomerase treatment, there was an increase in the number of blood cells (Bar et al., 2016).
Transplantation and Cychophosphamide
Another type of treatment being researched is a combination treatment with cychophosphamide, chemotherapy drug and then donor transplantation (DeZern et al., 2017). Researchers recruited 16 patients, aged 11-69 years old with severe aplastic anemia and were matched to bone marrow transplant between July 2011 through August 2016. They administer a patient's’ drugs to suppress the immune system and to prevent the donor marrow rejection. Then the patient receives bone marrow transplant. Afterwards, a high dose of chemotherapy drug called cyclophosphadmide is given to the patient for about a year. Researchers monitored the red and white blood cell and platelet count. The results reveal that the count for each had returned to normal without the need for blood transfusion. Researcher hypothesize that the reason the treatment is successful is because the drug destroys the patient's diseased immune cells but does not harm the donor’s blood cells therefore allow for disease free blood cells to survive in the patient (DeZern et al., 2017).
Quality of Life
Fatigue is one of the common symptoms of myelodysplastic syndrome (MDS), aplastic anemia (AA) and paroxysmal nocturnal hemoglobinuria (PNH) along with pain, depression, anxiety and stress. This can have a large impact on the quality of life of an individual. A study done by Escalante et al. looked into quality of life of individuals with MDS, AA and PNH. The average fatigue score of the patients was 28 (range 1-52) and the quality of life score was 67 (range 10-104) specifically for those with AA. The higher values of the ranges were indicative of high fatigue or better quality of life. Popular management strategies people used were preserving energy, exercise, and naps; the most popular strategy was preserving energy. (Escalante et al., 2015). People that are diagnosed with AA may be more limited to certain activities due to their increased amount of fatigue compared to those without AA.
Powerpoint Presentation aplastic_anemia_ppt._.pptx
References
Aplastic Anemia and MDS International Foundation. Causes. (2016). Retrieved October 25, 2017, from http://www.aamds.org/diseases/aplastic-anemia/causes Aplastic Anemia and MDS International Foundation. Types. (2016). Retrieved October 25, 2017, from http://www.aamds.org/diseases/aplastic-anemia/types
Bacigalupo, A. (2008). Treatment strategies for patients with severe aplastic anemia. Bone Marrow Transplantation, 42. doi:10.1038/bmt.2008.113
Bar, C., Povedano, J., Serrano, R., Benitez-Buelga, C., Popkes, M., & Formentini, I. et al. (2016). Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood, 127(14), 1770-1779. http://dx.doi.org/10.1182/blood-2015-08-667485
Braunstein, M.E. (2016) Aplastic Anemia. Retrieved 28 October 2017, from https://www.merckmanuals.com/en-ca/professional/hematology-and-oncology/anemias-caused-by-deficient-erythropoiesis/aplastic-anemia
Cuglievan, B., DePombo, A., & De Angulo , G. (2017). Aplastic anemia: the correct nomenclature matters. The Hematology Journal , 101 (9).
DeZern, A. E., & Brodsky, R. A. (2011). Clinical management of aplastic anemia. Expert Review of Hematology, 4(2), 221–230. http://doi.org/10.1586/ehm.11.11
DeZern, A., Zahurak, M., Symons, H., Cooke, K., Jones, R., & Brodsky, R. (2017). Alternative Donor Transplantation with High-Dose Post-Transplantation Cyclophosphamide for Refractory Severe Aplastic Anemia. Biology Of Blood And Marrow Transplantation, 23(3), 498-504. http://dx.doi.org/10.1016/j.bbmt.2016.12.628
Escalante, C. P., Chisolm, S., Song, J., Richardson, M., Ellen, S., Lam, T., et al. (2015). Fatigue, quality of life and related symptoms: patient reported outcomes in myeloplastic syndrome, aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood , 126 (23).
Guo, Y., Kartawinata, M., Li, J., Pickett, H., Teo, J., & Kilo, T. et al. (2014). Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood, 124(18), 2767-2774. http://dx.doi.org/10.1182/blood-2014-08-596445 Health 24 . (n.d.). The seven types of anemia. Retrieved October 25, 2017, from Health 24: http://www.health24.com/Lifestyle/Your-Blood/Anaemia-20130216-2
How Is Aplastic Anemia Diagnosed? - NHLBI, NIH. (2017). Nhlbi.nih.gov. Retrieved 28 October 2017, from https://www.nhlbi.nih.gov/health/health-topics/topics/aplastic/diagnosis
How Is Aplastic Anemia Treated? - NHLBI, NIH. (2017). Nhlbi.nih.gov. Retrieved 28 October 2017, from https://www.nhlbi.nih.gov/health/health-topics/topics/aplastic/treatment
Hussain, S. K. (2017, April 03). Aplastic Anemia and MDS Awareness Week: Let's Fight Against Anemia. Retrieved October 19, 2017, from https://www.consumerhealthdigest.com/health-awareness/aplastic-anemia-and-mds-awareness-week.html
Killick SB, Win N, Marsh JC, et al. (1997) Pilot study of HLA alloimmunization after transfusion with pre-storage leucodepleted blood products in aplastic anaemia. Br. J. Haematol. 97(3), 677–684.
Kojima, S. (2016). Why is the incidence of aplastic anemia higher in Asia? Retrieved October 24, 2017, from http://www.tandfonline.com/doi/full/10.1080/17474086.2017.1302797
Laundy GJ, Bradley BA, Rees BM, Younie M, Hows JM. (2004) Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 44(6), 814–825.
Mandal, A. (2013). What is hematology? Retrieved October 25, 2017, from News Medical Life Sciences : https://www.news-medical.net/health/What-is-Hematology.aspx
Marsh J, Socie G, Tichelli A, et al. (2010) Should irradiated blood products be given routinely to all patients with aplastic anaemia undergoing immunosuppressive therapy with anti-thymocyte globulin (ATG)? A survey from the European Group for Blood and Marrow Transplantation Severe Aplastic Anaemia Working Party. Br. J. Haematol. 150(3), 377–379.
Montané, E., Ibáñez, L., Vidal, X., Ballarín, E., Puig, R., García, N., . . . Catalan, A. N. (2008). Epidemiology of aplastic anemia: a prospective multicenter study. Retrieved October 24, 2017, from https://www.ncbi.nlm.nih.gov/pubmed/18322256
Myelodysplastic Syndrome - Types, Causes, Symptoms, Diagnosis, Treatment & Prognosis. (2017, May 31). Retrieved October 19, 2017, from http://www.medindia.net/patientinfo/myelodysplastic-syndrome.htm
N. (2016, February 15). Getting to know Aplastic Anemia. Retrieved October 19, 2017, from http://nursingcrib.com/nursing-notes-reviewer/getting-to-know-aplastic-anemia/
National Heart, Lung and Blood Institute. (2012). What is aplastic anemia? Retrieved October 24, 2017, from National Heart, Lung and Blood Institute: https://www.nhlbi.nih.gov/health/health-topics/topics/aplastic
National Heart, Lung, and Blood Institute. (2012). What causes aplastic anemia? Retrieved October 18, 2017, from https://www.nhlbi.nih.gov/health/health-topics/topics/aplastic/causes
Skalak, R., Tozeren, A., Zarda, R., & Chien, S. (1973). Strain Energy Function of Red Blood Cell Membranes. Biophysical Journal, 13(3), 245-264. doi:10.1016/s0006-3495(73)85983-1 Socie G, Henry-Amar M, Bacigalupo A, et al. (1993) Malignant tumors occuring after treatment of aplastic anemia. N. Engl. J. Med. 329, 1152–1157.
Solomou, E., Gibellini, F., Stewart, B., Malide, D., Berg, M., & Visconte, V. et al. (2007). Perforin gene mutations in patients with acquired aplastic anemia. Blood, 109(12), 5234-5237. http://dx.doi.org/10.1182/blood-2006-12-063495
Stemple, D. (2009). Faculty of 1000 evaluation for Hematopoietic stem cell development is dependent on blood flow. F1000 - Post-publication peer review of the biomedical literature. doi:10.3410/f.1161543.623346
Types. (2017). Aplastic Anemia and MDS International Foundation. Retrieved 28 October 2017, from http://www.aamds.org/diseases/aplastic-anemia/types
Young, N. and Kaufman, D. (2008). The epidemiology of acquired aplastic anemia. Retrieved October 26, 2017, from http://www.haematologica.org/content/93/4/489#sec-1