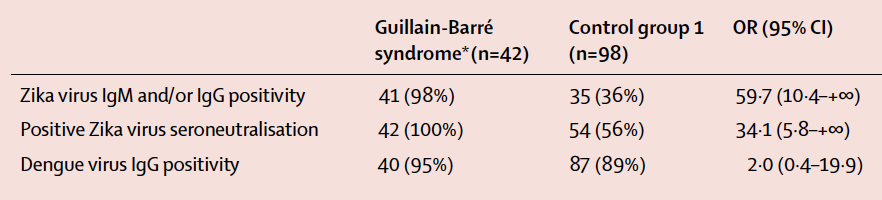Table of Contents
Zika Virus
Zika Virus PowerPoint
Zika Virus 101: What is Zika Virus?
Zika Virus (ZIKV) is among one of the most recent virus to capture international headlines and the attention of several health Organizations in recent times. The most recent outbreak of 2015 in the Americas were instrumental in the discovery of the link between ZIKV and the neurological diseases Guillain-Barre Syndrome in adults and microcephaly in infants born to infected mothers. ZIKV is an arbovirus of the family flaviviridae and is closely related to other flaviviruses such as yellow fever, dengue, west nile virus and Japanese encephalitis virus. The first ever discovery of ZIKV was in 1947 from the serum of a Rhesus monkey (CDC, 2018). One year later ZIKV was also isolated from the solution of a ground up Aedes africanus mosquito captured from the Zika forest in Uganda. 1952 saw the first ever recorded human infection through the detection of ZIKV neutralizing antibodies. Arguably ZIKV has two major geographical lineages, the African and the Asian lineages. The African ZIKV is characterised by a life cycle that predominantly resides in apes with the occasional human host whereas the Asian ZIKV lineage on the other hand almost exclusively resides in human hosts (Nayak et al 2016).
Epidemiology
There have been sporadic cases and serological evidence of ZIKV infection in Africa and Asia since its first discovery in 1948. This documentation of ZIKV activity is evidence that ZIKV was active in several African and Asian countries prior to the Pacific region outbreak and more recently the outbreak witnessed in the Americas. The serological study of 1954 carried out in French Equatorial Africa successfully isolated ZIKV antibodies in 0.5% of the population. Between the span of 1971 to 1975 another study identified ZIKV neutralizing antibodies in 38% of individuals and finally in 1977 a hospital in Java Indonesia confirmed ZIKV infection in 3.1% of febrile patients (Paixao et al, 2016).
Zika flew under the radar after the confirmed cases in Java in 1977/8. Essentially from then on there were no publications on ZIKA until the Yap Island outbreak of 2007. The Yap outbreak of 2007 also marked the first time Zika was detected in Oceania. With a population of 10,000 people it was thought that 1 in every 5 people had manifested symptoms of Zika virus, however there were no reported fatality related to Zika (Paixao et al, 2016). Following the Yap Island outbreak of 2007, there were only 4 studies published on Zika from 2008 to 2013 and in that time frame there were only 4 cases of Zika infection. Two of those cases were reported in Cambodia and the other two were reported in Colorado, when two scientist acquired Zika from Senegal and returned home to Colorado with one of the scientist transferring it to his wife via semen. No further outbreaks were reported until the outbreak of French Polynesia (Paixao et al, 2016). During this outbreak there was an increase in the number of reported neurological and autoimmune complications such as 42 cases of Guillain Barre Syndrome (GBS). On May 7th 2015 the Pan American Health Organisation (PAHO) confirmed the first case of ZIKV infection and with the Zika outbreak of 2015 came a rise in the reported cases of microcephaly (CDC, 2018). This warranted a retrospective study on the French Polynesia outbreak, which resulted in the identification of 17 cases of malformations in the foetuses and newborns of women who were pregnant during the Zika outbreak on the Island. This discovery changed the perception of Zika as a harmless old disease. According to estimates from the Brazillian Ministry of Health, there were approximately 1.3million cases of Zika in early 2015. Unlike previous outbreaks, there were 46 confirmed deaths in Brazil linked to Zika virus (Paixao et al, 2016).
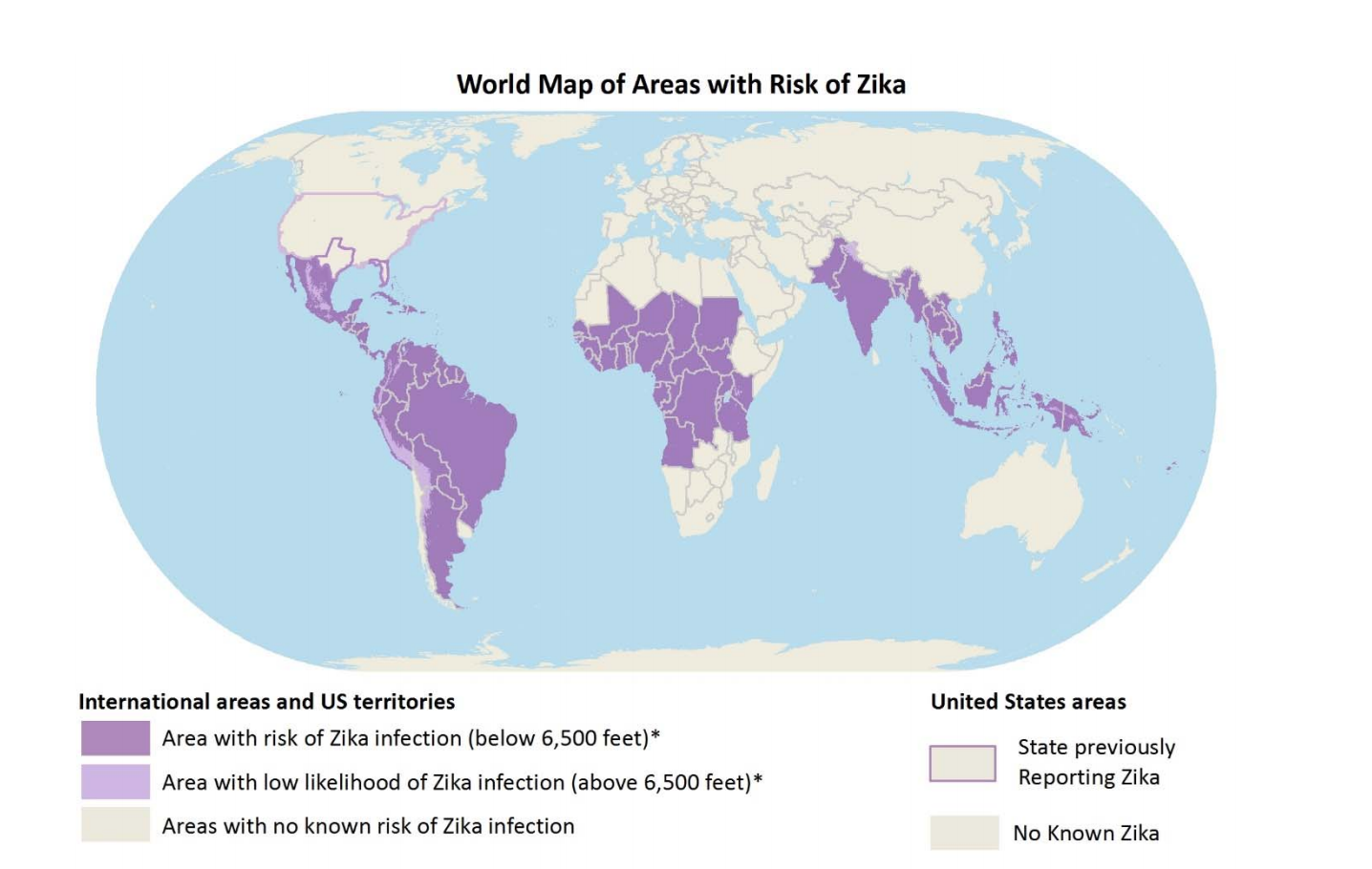
As of 2016, 27 countries in the Americas as seen in Figure 1 have reported Zika infections, which includes Colombia, Guatemala, Mexico, Panama, Paraguay, Venezuela, El Salvador, Honduras and Martinique, Bolivia, Guyana, Ecuador, Guadeloupe, Puerto Rico, Saint Martin and Haiti. Molecular studies later showed that the ZIKV from French Polynesia and Brazil were the same strain and clearly from the Asian lineage. It is believed that a traveller from French Polynesia was the source of the ZIKV outbreak in Brazil (Paixao et al, 2016).
Transmission
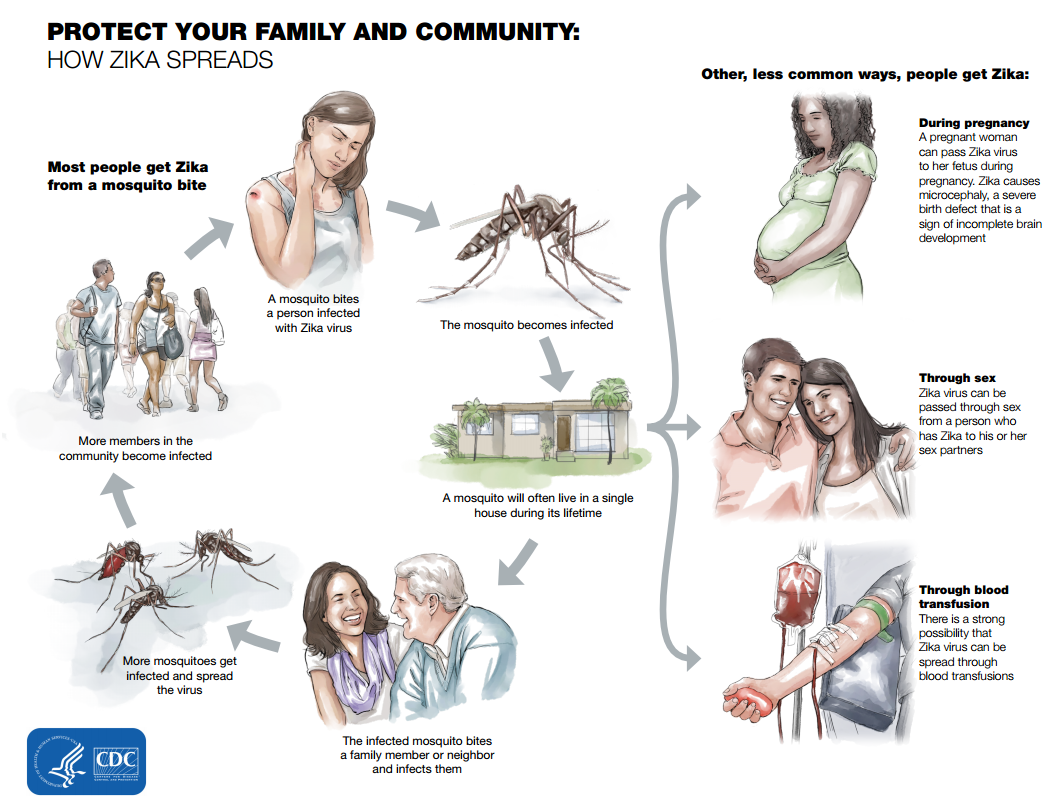
The natural vertebrate host of Zika virus in vertebrates were primarily monkeys. The infection normally spreads in a mosquito-monkey-mosquito cycle with the occasional infection of humans. Prior to the current pandemic that began in 2007, Zika spill over to humans rarely occurred. The primary mode of ZIKV transmission in humans is through the bite of an infected Aedes aegypti mosquito as summarised in Figure 2, other modes of transmission includes transmission through sexual contact, blood transfusions, laboratory exposures and maternal to fetal transmission through pregnancy. The transmission of ZIKV infection is so effective that by the end of 2016 more than 50 countries have reported cases of active cases of transmission though one or more means (CDC, 2018).
Although the primary mode of transmission is thought to be through the bite of the female Aedes aegypti mosquito, ZIKV has been isolated in about 6 other mosquitoes in the Aedes genus and 5 other species of mosquitoes. The truth is that the true extent of Zika vectors remains unknown (Ayres 2016)(Hayes, 2009). These posses a significant problem as one of the species of mosquitoes capable of carrying ZIKV has rapidly expanded across all continents. There have been confirmed cases of sexual transmission by both males and females in 6 countries including the USA, but there have been no confirmed cases of transmission through blood transfusions in USA although there are suspected cases in Brazil. ZIKV can also be transmitted through laboratory exposure and through mother to child during all stages of pregnancy. To date there have been only three reported case of ZIKV transmission through laboratory exposure, one in the USA and two in Italy. The recent discovery of ZIKV RNA in most bodily fluids suggest the presence of other modes of transmission (CDC 2018).
Pathogenesis
Our knowledge on ZIKV pathogenesis is severely limited due to the extreme lack of sufficient publications on ZIKV prior to the outbreak of 2015 in Brazil, as a result most literature with regards to mechanisms of ZIKV are inferred from research on other Arboviruses of the same family. Despite the limited publications there are several hypothesised mechanisms of ZIKV infection based on the knowledge that ZIKV has been shown to activate different pathways based on the type of tissue/Organ of infection. Ultimately ZIKV results in cell death irrespective of tissue/organ of infection. We will focus on the brain and nervous system.
Brain and Nervous System
A study by Nayak et al 2016 using mouse models identified radial glial cells, a type of neural progenitor cells as the primary target of ZIKV in fetal mouse brains. In this study the Asian ZIKV strain was injected into immunosupprescent mice and the effects were observed. Upon injection ZIKV was shown to target neural progenitor cells. They were also shown to prevent the proliferation and differentiation of cortical neural progenitor cells. Although genes that regulate organ development were shown to be downregulated, a gene analysis also showed that genes involved in apoptosis pathways and immune response such as cytokine production were upregulated. This study demonstrated the persistent replication of ZIKV up to twenty eight days after the initial infection (Nayak et al 2016). An infected cell is characterised by rounding, pyknosis and caspase activation. Several molecular pathways have been put together to explain the mechanisms of ZIKV infection one of which includes the cytokine receptor AXL which has been shown to mediate ZIKV infection. Most cells in the body are enriched with AXL kinases especially the cells along the pathway of ZIKV infection which includes the skin, the lymph node, micro-capillaries, microglial and cortical astrocytes. This enables the rapid expansion of ZIKV. ZIkV then signals the upregulation of AXL kinases resulting in reduced neural cell proliferation and cell death. ZIKV has also been shown to upregulate genes associated with autophagy and apoptosis such as Bmf1, Casp6 and can activate toll-like receptor 3 (TLR3) in human embryonic stem cell derived organoids which leads to the disregulation of genes involved in neurogenesis and apoptotic pathways resulting in the manifestation of clinical neurological symptoms (Nayak et al 2016).
Signs & Symptoms
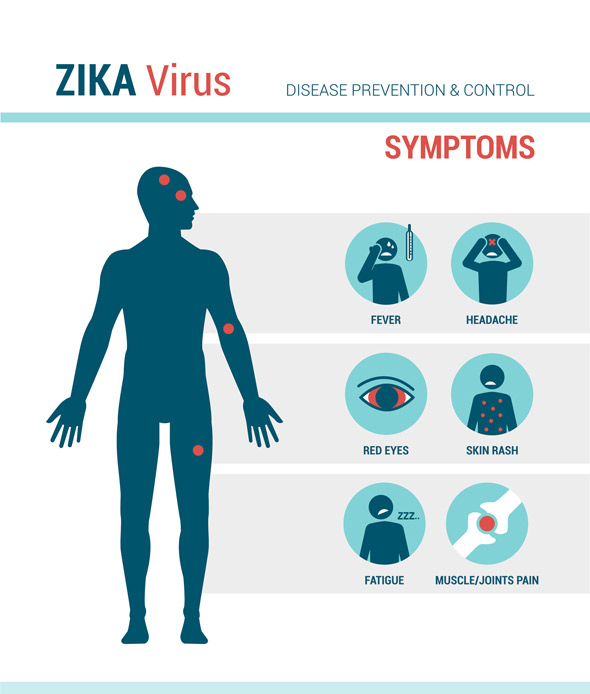
Those infected with Zika Virus may not experience symptoms or might have mild symptoms associated with the infection. The typical symptoms include a fever, rash (flat and red covered by small bumps), headaches, joint pain, conjunctivitis, muscle pain, weakness and an overall lack of energy. The incubation period, from the time of exposure to the presentation of symptoms, is unclear but is usually around three to seven days. The symptoms are mild and can last from several days to a week. Individuals are not usually sick enough to go to the hospital and thus might not even realize they have been infected. Only one in four people are believed to develop symptoms and the symptoms are similar to other viruses spread by mosquitos such as Dengue and Chikungunya virus.
Testing
Zika Virus is present in the blood of an infected person for roughly a week. Testing is recommended if you develop symptoms or if you have traveled recently to an area at risk. It is also recommended if you've had sexual contact with someone who has travelled to an area at risk, especially important if you are pregnant. Blood and urine tests are the primary means to confirm Zika Virus infection and are used for diagnosis. Other means to diagnose Zika Virus include semen, cerebrospinal fluid (CSF) and amniotic fluid, but are not as common. To test blood or urine of someone who has had symptoms for less than seven days, you can use nucleic acid testing (NAT) via RT-PCR to look for targets on the surface of the Zika Virus. For symptoms that are present greater than 7 days, you can confirm diagnosis with serology by looking for IgM.
Treatment
There is no specific treatment, either medicine or vaccine, for Zika Virus. Instead of a specific treatment, Zika Virus care typically focuses on addressing the symptoms as a result of the virus. For example, rest and fluids are used to prevent dehydration. Tylenol (Acetaminophen) is used to help with fever and pain related symptoms. However, one should not take aspirin or other non-steroidal anti-inflammatory medications due to the possibility of having Dengue Virus. Dengue Virus symptoms are very similar to Zika Virus and if an individual with Dengue Virus was to take non-steroidal anti-inflammatory medications, they are at risk for sever bleeding or hemorrhage.
Zika Virus Preclinical Studies
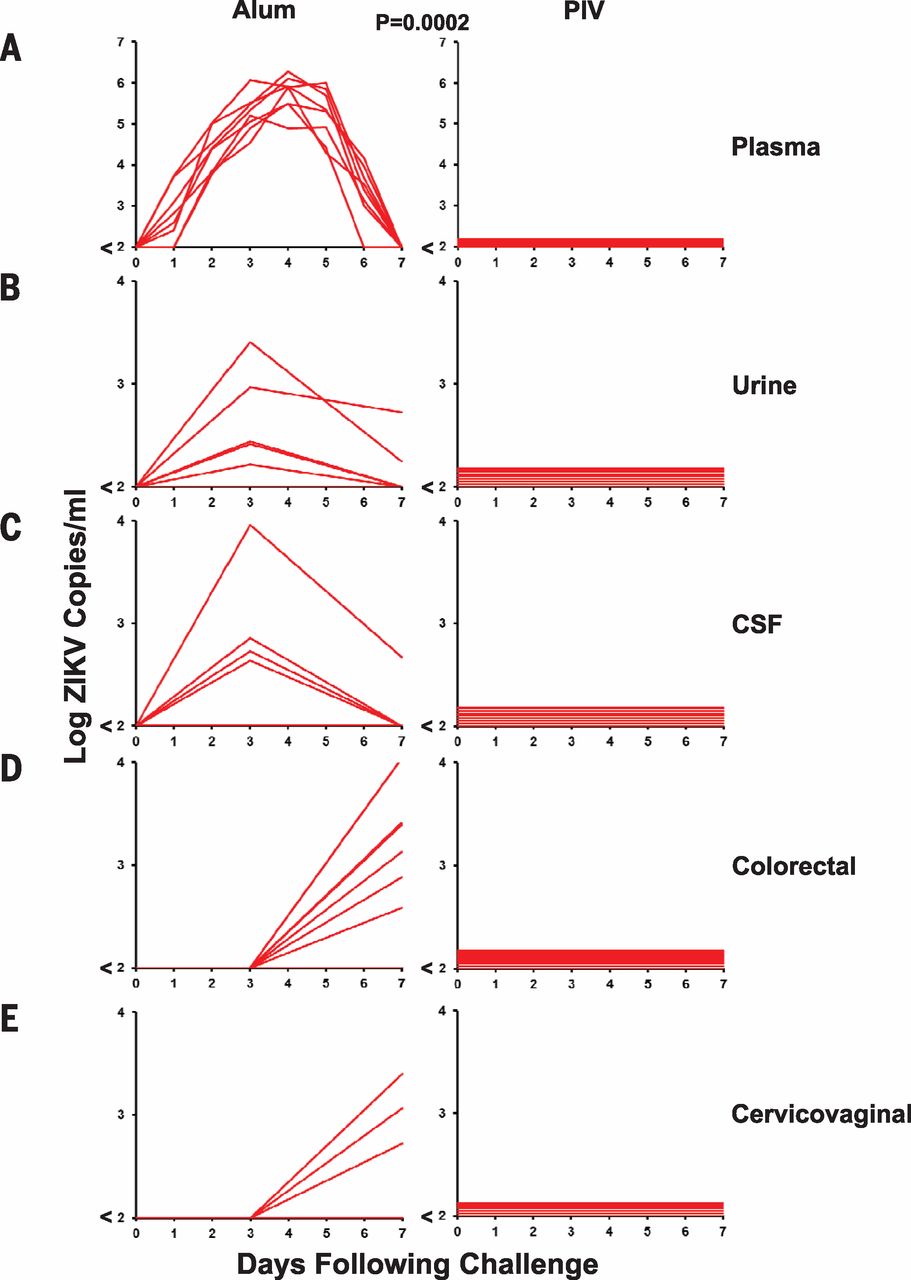
Several studies, such as a study conducted by Abbink et al. (2016), have shown promising results in the preclinical phase of vaccine development. Abbink et al. (2016) immunized eight rhesus monkeys with a Zika Purified Inactivated Virus (PIV) and eight with aluminum potassium sulfate as a control at both zero and four weeks. Upon analysis, it was determined that all eight Rhesus monkeys who received the Zika PIV developed Zika specific binding antibodies according to enzyme-linked immunosorbent assays and microneutralization assays (MN50) whereas the other eight Rhesus monkeys did not develop the antibodies. The researchers then infected both groups with viral particles of Zika-BR or Zika-PR with an n=4 per treatment. After measuring the viral load with RT-PCR, researchers determined that the control monkeys showed ZIKV-specific MN50 tigers increase after the Zika Virus challenge. Control monkeys also showed six to seven day viremia with peak loads on days three to five. The virus was detected in the urine, CSF as well as colorectal secretions as seen in Figure 4. In contrast, PIV vaccinated monkeys showed complete protection against ZIKV challenge as no virus was present in any of the detection methods.
Researchers did other studies using adoptive transfer of vaccine elicited antibodies (IgG) and at high doses they protected from Zika virus. Researchers also tested DNA plasmid based vaccines on Rhesus monkeys which also showed protection against Zika Virus. They determined that there were no adverse side effects and that there were also similar results with the PIV vaccine seen in mice. The only side note the researchers mentioned was that studies needed to be performed to evaluate cross-reactivity with antibodies to dengue viruses and other similar viruses.
Zika Virus Clinical Studies
As a result of the success of several preclinical studies, clinical trials have been underway to evaluate the effectiveness of potential Zika Virus vaccines. In 2016, the National Institute of Allergy and Infectious Disease (NIAID) conducted a phase 1 clinical trail aimed at evaluating the safety of a Zika vaccine and its ability to generate an immune response. The researchers recruited 80 healthy volunteers between the ages of 18-35 in the United States. The vaccine included a DNA Plasmid with genes coding for proteins expressed on the surface of the Zika virus. The purpose was to generate an immune response against these proteins that were created which would be slightly better than introducing a live attenuated version of the Zika Virus. They split the participants into four groups of 20 and initially vaccinated all individuals. Half of the individuals got another vaccine at eight or 12 weeks and the remaining participants got an additional two vaccinations. One group of 20 got the second vaccine at week four and the third at week eight and the last group of 20 got the second vaccine at week four and the third at week 20. All individuals in the study were followed up on at 44 weeks and the results indicated that the vaccine was safe and produced neutralizing antibody responses (NIAID, 2016).
As a result of this success, the researchers at NIAID moved on to a phase 2 clinical trail with 2490 healthy participants in areas with confirmed Zika Infection such as South American countries to further evaluate the safety of the vaccine. They also set out to determine a dosage range and if the vaccine can prevent disease caused by a natural ZIKA infection. This study also utilized a DNA plasmid vaccine that contained genes coding for proteins found on the surface of the Zika virus. The study consisted of two parts, Part A and Part B. Part A included 90 men and women in affected areas with Zika virus and all will get vaccinated three times four weeks apart. All participants were randomly assigned to the standard or a higher dose which both aimed at determining the safety of the vaccine, dosing ranges and if the vaccine can stimulate an immune response. Part B consisted of a double blinded study with 2400 men and women to determine if the vaccine can protext agaisnt Zika virus if exposed to the virus naturally. The participants were randomly assigned to receive the vaccine or the placebo three times spread four weeks apart similar to Part A. The researchers plan on following the participants for two years to track the effects. Until 2019, the researchers will guide volunteers on preventative measures and then will compare Zika virus rates present in both groups.
Risk and Health Effects
You are at risk of being infected with Zika virus if you travel to any countries or area with reported Zika-carrying mosquitoes. Mosquitoes carrying Zika virus are active during both day and night hours, however if you are travelling at elevations greater than 2,000 metres your risk is generally low as mosquitoes do not live at high altitudes. Additionally, the risk to Canadians is low as Zika-carrying mosquitoes cannot survive in Canada's cold climate (Government of Canada, 2017).
The risk for a person infected with Zika virus to actually develop symptoms is 1 in 4 (Government of Canada, 2017), which may include fever, fatigue, skin rash, among other physical symptoms. Rarely, studies have shown that Zika virus infection is associated with Guillain-Barre Syndrome (GBS), a autoimmune disorder that damages an individual's neurons (Cao-Lormeau et al., 2016; CDC, 2018).
Finally, Zika virus infection poses the greatest risk to a developing fetus. Since Zika can remain in the blood up to 1 week after initial infection and in the semen up to 6 months, special precautions must be taken to ensure the safety of a new or current pregnancy. Pregnant women who are exposed to the Zika virus or women who conceive a child whilst infected put their fetus at risk for severe birth defects. Specifically, the group of birth defects associated with Zika virus infection is called Congenital Zika Syndrome. Congenital Zika Syndrome is characterized by defects such as microcephaly, decreased brain tissue, ocular damage, club foot, and increased muscle tone that limits movement (CDC, 2018; Government of Canada, 2017).
Guillain-Barre Syndrome (GBS)
Retrieved Jan 23 from https://www.youtube.com/watch?v=4omfTbiB0kk
Guillain-Barre Syndrome (GBS) is a rare autoimmune disease that affects neurons. Specifically, the myelin coating around axons is damaged and the conduction of signals between nerve cells is compromised.
A recent study by Cao-Lormeau et al. (2016) published in The Lancet presented considerable evidence linking GBS with previous Zika virus infection. The researchers presented a case study involving 42 individuals with confirmed GBS during a massive Zika virus outbreak in French Polynesia between 2013 and 2014. Blood samples were taken from patients with confirmed GBS (n=42) and patients without GBS (control; n=98). The researchers used various techniques to detect antibodies against Zika virus (IgM/IgG) in both groups of patients. Evidence of previous Zika virus infection, demonstrated by the presence of Zika antibodies, was shown for 41 (98%) patients in the GBS group compared to 35 (36%) of patients in the control group (Figure 5; p<0.0001). In addition, a neutralizing response against Zika virus was seen in 42 (100%) of the GBS patients and 54 (56%) of the control group patients (Figure 5; p<0.0001). This data presented by Cao-Lormeau et al. (2016) exhibits a significant association between previous Zika virus infection and GBS.
Microcephaly
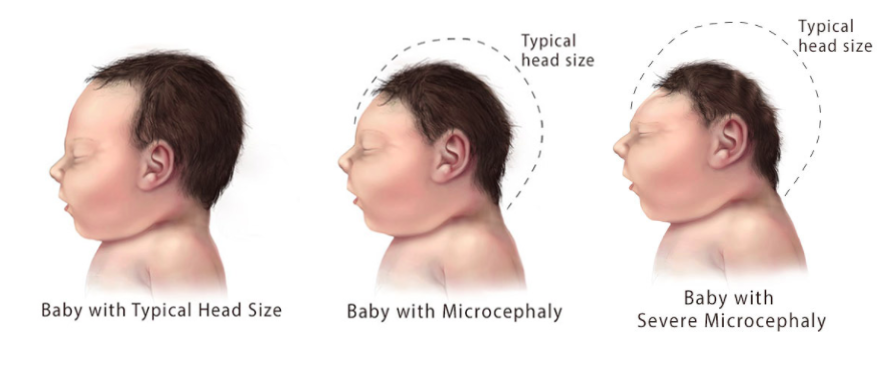
Microcephaly is a birth defect associated with Congenital Zika Syndrome. Babies with microcephaly have a smaller head circumference than age and sex-matched patients. The decreased head circumference is due to improper or lack of brain development during pregnancy (CDC, 2018). Severe microcephaly (Figure 6) is an extreme form of the condition where the baby's head circumference is much smaller (~1st percentile) than babies of the same age and sex (~3rd percentile) (CDC, 2018).
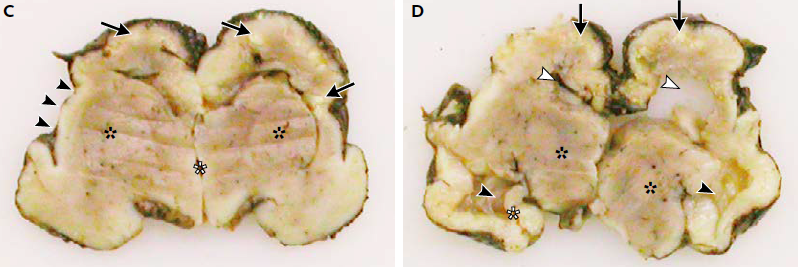
A case study published by Mlakar et al. (2016) in The New England Journal of Medicine described the morphology of a baby with confirmed severe microcephaly. The mother, who had been infected with Zika virus, requested termination of the pregnancy at 32 weeks. At the time of termination the fetus' head circumference was 26cm (1st percentile). An autopsy performed on the fetal brain showed clear degeneration, calcifications, malformation and incomplete development of important structures (Figure 7), resulting in the small head circumference characteristic of microcephaly.
Figure 7: Autopsy of fetal brain. Panel C shows white calcifications and loss of gyration in cortex (Black arrows), open sylvian fissures (Black arrowheads), and poorly delineated basal ganglia (Black asterisks). Panel D shows dilated lateral ventricles and collapsed left ventricle (White arrowheads), thalami are well developed (Black asterisks) along with the hippocampus (White asterisks), however contralateral structure failed to develop. (Modified from Mlakar et al., 2016)
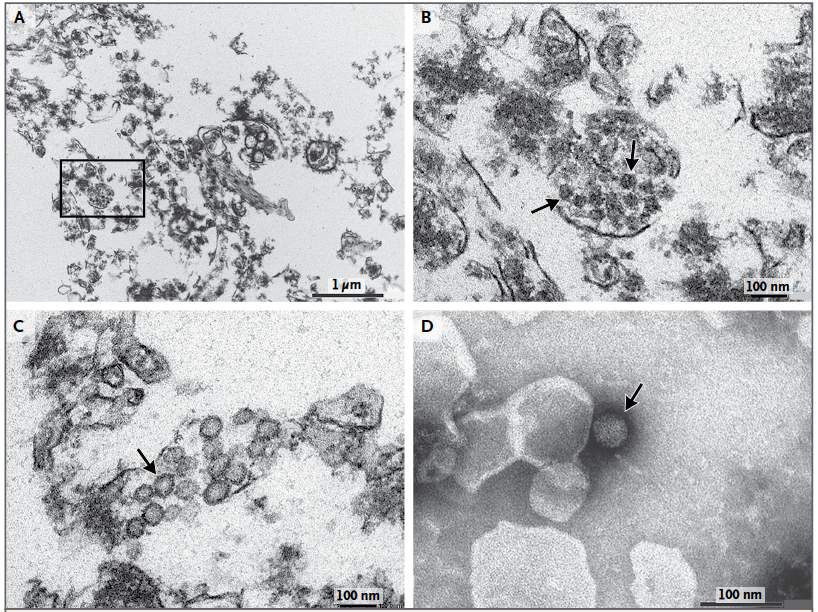
In addition, Mlakar et al. (2016) used electron microscopy to image ultra-thin sections of the fetal brain and staining of viral particles in order to visualize the presence of Zika virus in the fetal brain tissue. Imaging showed dense clusters of viral particles among the damaged brain tissue, as well as the presence of active viral replication (Figure 8).
Figure 8: Electron microscopy of ultra-thin sections of the fetal brain and staining of viral particles. Panel A shows dense clusters of virions amongst damaged brain cells. Panel B is a magnified image of Panel A, clearly showing the virion clusters. Panel C shows viral particles with bright interiors indicative of viral replication. Panel D is a negative staining of a virion showing morphological characteristics consistent with Zika virus (Modified from Mlakar et al., 2016)
Prevention Strategies
Planning a Healthy Pregnancy
In order to ensure a healthy pregnancy for both the woman and the fetus, both the woman and her partner should refrain from entering areas with documented Zika virus, especially those in which transmission by mosquitoes is likely to occur (CDC, 2018). Since the Zika virus can be transmitted through sexual intercourse, both the partner and the woman must take precautionary measures.
If a woman and/or her partner live(s) in an area infected by Zika virus, they must take steps in order to prevent mosquito bites, as well as practice safe sex (CDC, 2018).
Vector-borne Transmission
There are several measures that can be taken to decrease the risk of exposure to the Zika virus and hopefully prevent the spread of the disease. Since there is no vaccine yet available for the disease, global health organizations and government healthcare departments have been advocating the implementation of numerous prevention methods. The methods of prevention can be divided into two subgroups: protection against mosquitoes and prevention of sexual transmission.
According to the CDC (2017), the most effective methods to protect against mosquitoes include wearing clothes that cover up as much of the body as possible, preferably in lighter colours. Personal clothing items can also be treated with permethrin, an effective insecticide. The use of insect repellent is highly encouraged even after returning from a Zika active region to avoid the risk of spreading the disease to other mosquitoes (CDC, 2017). Insect repellent containing DEET, IR3535 or icaridin is recommended. In addition, physical barriers, such as mosquito nets and window screens, should also be used to prevent mosquito bites. If these are not available, doors and windows should remain closed as much as possible. Potential breeding grounds nearby and within the home should be covered, emptied or cleaned (namely buckets, pots, gutters, used tyres, etc.) to prevent mosquito breeding. Finally, make sure to implement these prevention methods for those who cannot do so themselves, i.e. young children and the elderly.
Sexual Transmission
In terms of protecting against sexual transmission, the prevention methods depend on your sex and whether you have been exposed to an active Zika region firsthand. The main concern with the sexual transmission of the Zika virus is the role that it plays in the developmental deficits exhibited by a newborn child – most notably, microcephaly.
For women returning from an active Zika region, it is recommended to wait a minimum of 8 weeks before trying to conceive a child with your partner (unless a male partner has been exposed to an active Zika region, in which case it is best to adhere to the male prevention methods) as it takes 2 months to clear the infection in women (WHO, 2016). It is also important to consistently use condoms and other appropriate contraceptives or abstain from sex for a minimum of 8 weeks, especially if you are pregnant.
For men returning from an active Zika region, safe sex practices, such as wearing a condom, should be practiced for a minimum period of 6 months, as the virus can survive for 6 months in the semen of infected males (Government of Canada, 2017). Couples should also wait 6 months before trying to conceive a child. Any scheduled semen donations should be postponed for a minimum of 6 months (Government of Canada, 2017).
For men and women that have both travelled to active Zika regions wishing to donate blood, tissues, organs, or cells, it is recommended to wait a minimum of 21 days before making donations as it may take up to 21 days to become symptomatic (Government of Canada, 2017).
References
Abbink, P., Larocca, R. A., De La Barrera, R. A., Bricault, C. A., Moseley, E. T., Boyd, M.,… Barouch, D. H. (2016). Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science, 353(6304), 1129-32. doi:10.1126/science.aah6157
Ayres, C. (2016). Identification of Zika virus vectors and implications for control. The Lancet Infectious Diseases, 16(3), 278-279. http://dx.doi.org/10.1016/s1473-3099(16)00073-6
Baud, D., Gubler, D. J., Schaub, B., Lanteri, M. C., & Musso, D. (2017). An update on Zika virus infection. The Lancet, 390(10107), 2099-2109.
Cao-Lormeau, V., Blake, A., Mons, S., Lastere, S., Roche, C., Vanhomwegen, J., Dub, T.,… Ghawche F. (2016). Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. The Lancet, 387, 1531-1539. http://dx.doi.org/10.1016/S0140-6736(16)00562-6
Centers for Disease Control and Prevention (CDC). (2018, Jan 11). Zika Virus. Retrieved from https://www.cdc.gov/zika/index.html
Centers for Disease Control and Prevention (CDC). (2018, Jan 11). Zika Virus – Protect Yourself and Others. Retrieved from https://www.cdc.gov/zika/prevention/protect-yourself-and-others.html
Government of Canada. (2017, March 8). Risks of Zika Virus. Retrieved from https://www.canada.ca/en/public-health/services/diseases/zika-virus/risks-zika-virus.html
Government of Canada. (2017, March 8). Prevention of Zika Virus. Retrieved from https://www.canada.ca/en/public-health/services/diseases/zika-virus/prevention-zika-virus.html
Hayes, E. (2009). Zika Virus Outside Africa. Emerging Infectious Diseases, 15(9), 1347-1350. http://dx.doi.org/10.3201/eid1509.090442
Mlakar, J., Korva, M., Tul, N., Popovic, M., Poljsak-Prijatelj, M., Mraz, J, Kolenc M.,… Zupanc, T.A. (2016). Zika virus associated with microcephaly. New England Journal of Medicine, 374(10), 951-958.10.1056/NEJMoa1600651
National Institute of Allergy and Infectious Diseases. (2016, August 3). NIH Begins Testing Investigational Zika Vaccine in Humans | NIH: National Institute of Allergy and Infectious Diseases. Retrieved from https://www.niaid.nih.gov/news-events/nih-begins-testing-investigational-zika-vaccine-humans
National Institute of Allergy and Infectious Diseases. (2017, March 3). Phase 2 Zika Vaccine Trial Begins in U.S., Central and South America | NIH: National Institute of Allergy and Infectious Diseases. Retrieved from https://www.niaid.nih.gov/news-events/phase-2-zika-vaccine-trial-begins-us-central-and-south-america
Paixão, E., Barreto, F., da Glória Teixeira, M., da Conceição N. Costa, M., & Rodrigues, L. (2016). History, Epidemiology, and Clinical Manifestations of Zika: A Systematic Review. American Journal Of Public Health, 106(4), 606-612. http://dx.doi.org/10.2105/ajph.2016.303112
Public Health Agency of Canada. (2017, December 15). Zika virus. Retrieved from https://www.canada.ca/en/public-health/services/diseases/zika-virus.html
World Health Organization. (2016, September 6). Zika virus. Retrieved from http://www.who.int/mediacentre/factsheets/zika/en/