Table of Contents
Primary Systemic Amyloid Light-Chain (AL) Amyloidosis
PDF Presentation: pdf_-_final_version_ls_4m03_al_amyloidosis.pdf
PowerPoint Presentation: final_version_ls_4m03_al_amyloidosis.pptx
Introduction
What Is AL Amyloidosis?final_version_ls_4m03_al_amyloidosis.pptx:
History
To understand the history of AL Amyloidosis, it is necessary to investigate the history of the term, ‘amyloid’. This term was first used in 1854 by Rudolph Virchow to define extracellular accumulation in the liver (Nyirady, 2016). Thus, this was the first instance of this term being used in the same context as it is now presently. However, before Virchow coined this term, in 1838 a German botanist by the name of Mathias Schleiden used the term to refer to the, “normal amylaceous constituent of plants” (Nyirady, 2016). Interestingly, if one were to look further back into history, it becomes apparent that amyloids and the disease amyloidosis were known by several different names by doctors conducting post-mortem autopsies (Sipe, 2008). In these autopsies, abnormal deposits of amyloids in the liver or spleen were identified (Sipe, 2008). For example, even as far back as Nicolaus Fontanus in 1639, identified during an autopsy that a patient’s spleen had abnormal white stones (Kyle, 2001). Thomas Bartholin, Antoine Portal, and Jeremiah Wainewright are all other examples of doctors during different periods in history that all identified similar symptoms in patients or noticed similar characteristics when conducting autopsies (Kyle, 2001). Eventually, it was in 1856 when a doctor reported symptoms of something typical of primary amyloidosis (Kyle, 2001). This doctor was Samuel Wilks and he remarked on the autopsy of a 52-year-old man who died with, “lardaceous viscera” that the man’s kidneys were white (Kyle, 2001). Up to this point, there was no test to positively identify tissues that display amyloids (Beckerman, 2015). This changed in 1927 when Paul Divry and associates discovered that Congo red dye could be used to stain tissue samples suspected of containing amyloids (Beckerman, 2015). These tissue samples would glow green when viewed under polarized light (Beckerman, 2015). Finally, in 1959 Cohen and Calkins discovered that amyloidosis demonstrated a fibril structure using an electron microscope (Nyirady, 2016).
Classification
Systemic amyloidosis can be classified into 3 categories: primary systemic amyloidosis (PSA), amyloidosis associated with multiple myeloma and secondary systemic amyloidosis (Nyirady, 2016). Primary systemic amyloidosis is also known as AL Amyloidosis.
Epidemiology and Risk Factors/Causes
AL Amyloidosis is the most common type of Systemic Amyloidosis with an estimated incidence of 9 cases per-million inhabitants each year (Real de Asúa et al., 2014). In the USA there are approximately 1275-3200 new cases annually (Real de Asúa et al., 2014). These values however are not considered precise due to often un-diagnosis and misdiagnosis. A reported 56% of all systemic cases are classified as AL Amyloidosis (Nienhuis, Bijzet & Hazenberg, 2016). In the UK 65% of the Amyloidosis diagnosed patients have AL Amyloidosis while in China 93% are Amyloidosis(Desport et al., 2012).
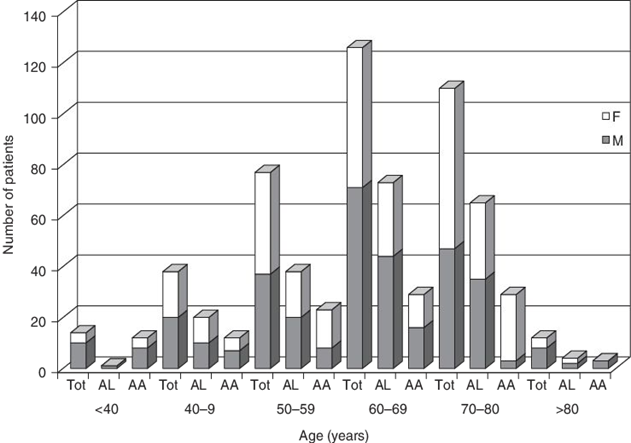
Figure 1: Patients age at time of diagnosis for AL/AA Amyloidosis along with patients sex.
AL Amyloidosis is a disease of adulthood with an incidence rate highest amongst adults aged 60 – 80 years old with the average age of diagnoses being 65 years (Real de Asúa et al., 2014). Only 10% of all patients are under the age of 50 and an even lower 5% of total patients are under the age of 40 (Real de Asúa et al., 2014). There have ever only been a handful of cases where the patient diagnosed with AL Amyloidosis was in their late 20’s. Although the reasoning to it is still unknown two-thirds of all patients are male. There has been no racial predilection reported in developing AL Amyloidosis (Nienhuis, Bijzet & Hazenberg, 2016).
AL Amyloidosis is not considered hereditary; it is not secondary to other diseases and cannot be passed on to others through aerosols/touch/sex. There are however a few families that have increases incidence to blood diseases which would include myeloma and AL Amyloidosis amongst others. This is not considered a norm (Desport et al., 2012).
Finally, the causes of patient developing AL Amyloidosis are not known and there is no known correlation with a person’s diet, activity, or stress levels (“Amyloidosis”, n.d.). However, some patients that have other diseases like, “multiple myeloma, Hodgkin's disease, certain tumors, and familial Mediterranean fever” do show a risk of having an association with amyloidosis (“Amyloidosis”, n.d.).
Signs and Symptoms
According to the Cedar-Sinai Cancer Institute (“Amyloidosis”, n.d.) the signs and symptoms of a patient potentially afflicted with Amyloidosis are hard to identify. This is because the disease is so rare and that many of the symptoms overlap with the characteristics displayed by patients with other diseases. Similarly, because Amyloidosis can affect different organs of the body, this too affects what symptoms a patient may display. Further, it is possible that a patient might not display any symptoms whatsoever although they may have the disease. Thus, all these factors contribute to the complexity of diagnosing this disease solely through signs and symptoms (“Amyloidosis”, n.d.).
Nevertheless, there are some key symptoms, which are generally associated with the disease (“Amyloidosis”, n.d.). These are:
“
- An enlarged liver
- An enlarged tongue
- An irregular heartbeat
- Diarrhea alternating with constipation
- Difficulty swallowing
- Dizziness or feeling faint
- Loss of weight
- Numbness or tingling in the hands or feet
- Severe fatigue
- Shortness of breath
- Skin changes
- Swelling of the ankles and legs
- Weakness…
- A burning sensation
- Bowel obstruction
- Carpal tunnel syndrome
- Nervous system disrupted” (“Amyloidosis”, n.d.).
It is also important to note that if the disease is affecting the heart or kidney, the symptoms displayed may be more severe and will pertain to the respective organ, which is being affected (“Amyloidosis”, n.d.). Usually in these cases the disease is life threatening (“Amyloidosis”, n.d.).
Prognosis
http://circ.ahajournals.org/content/133/3/245
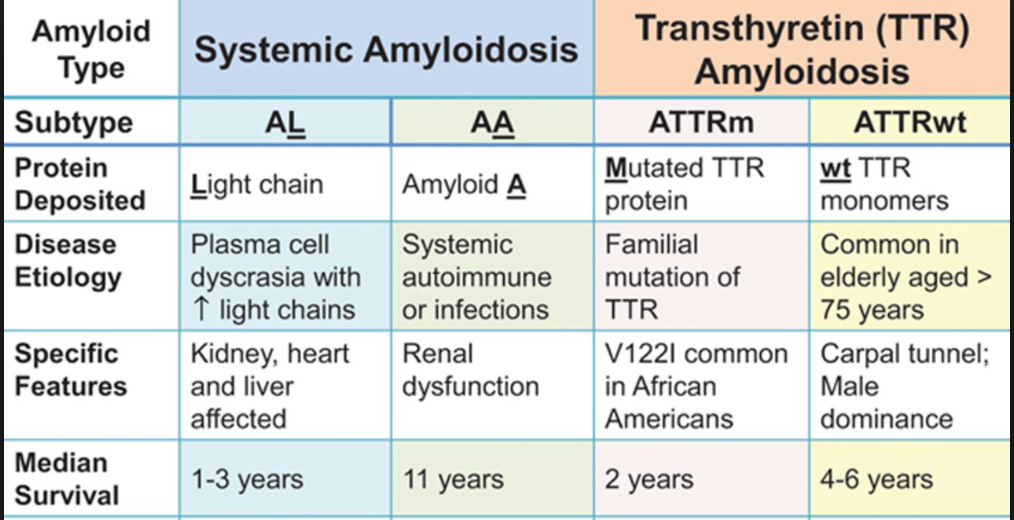
Table 1: The prognosis of different amyloidosis. (Liu, P.P., & Smyth, D., 2015).
If we would like know the prognosis of Amyloidosis, it is basically depends on the forms of amyloidosis and its response to the treatment. Also, vital organ involvement will have adversely effected on the life expectancy (Liu, P.P., & Smyth, D., 2015; Shiel, C.W., & Balentine, R. J., 2017; Papadakis, M.A., & McPhee, S. H., 2017).Median survival for AL (primary) amyloidosis is approximately 1-3 years. If patients do not treat the disease, it will slowly progress and become fatal. (Ma, G., & Ra, K., 1994; Tidy, C., 2014; Liu, P.P., & Smyth, D., 2015; Shiel, C.W., & Balentine, R. J., 2017). AL Amyloidosis is fatal in 80% of cases (Tidy, C., 2014).Sometimes, AL Amyloidosis will affect different parts of our body. If it affects our cardiac or autonomic nerve, people generally have survivals of 3 to 9 months; similarly, for Carpal tunnel syndrome or nephrosis, they have survivals of 1.5-3 years. In addition, if people have peripheral neuropathy, they may have survivals of 5 years by using multiple treatment (Papadakis, M.A., & McPhee, S. H., 2017). These data demonstrated the survival rates based on the patients who received the treatment.
Nowadays, for the patients who have AL Amyloidosis, if they can get treat by autologous hematopoietic stem cell transplantation properly, they have the median survival approaches 5 years now. Moreover. If patients get a complete hematologic remission, they approaches 10 years (Papadakis, M.A., & McPhee, S. H., 2017). Moreover, if patients have amyloidosis is related to the kidney, some researches illustrated that patients have dialysis and kidney transplantation; they will have further improved the prognosis (Tidy, C., 2014).
Targeted Organs:
http://www.bloodjournal.org/content/119/19/4343?sso-checked=true
Figure 2: Percentages referring to mortality rate (death within 3 years) upon development of AL Amyloidosis.
AL Amyloidosis can target a variety of organs. Mortality rates vary depending on the organ that the amyloid fibrils form within. The heart is the most sensitive to fibril formation and such mortality is very high if amyloid fibrils form. Figure 00 outlines some of the main organs that AL Amyloidosis target and the respected mortality rate that comes along with it. Every organ has a respectful threshold to the amount of amyloid it can withstand before losing functionality and failing. The heart and the kidney's have the lowest such threshold (Gertz et al., 2016).
Diagnosis
Patients who have unexplained proteinuria (presence of excess proteins in the urine), cardiomyopathy (diseases of the heart muscle), neuropathy, or hepatomegaly and in patients with multiple myeloma that has atypical manifestations all should be considered for having AL Amyloidosis (Sanchorawala, 2006). Diagnosis requires two criteria, the first being a demonstration of amyloid in the tissue and the second being a demonstration of a plasma cell dyscrasia (Sanchorawala, 2006). In about 15-20% of patients the most common skin signs include petechieae, purpura and ecchymoses (Nyirady, 2016). Petechieae are considered to be small purpura where purpura are areas of discolouration on the skin or mucous membranes that comes about because of hemorrhages from small blood vessels (Ngan, 2005). Ecchymoses also known as bruises are larger extravasations of blood (Ngan, 2005). These skin signs all occur spontaneously or after minor trauma (Nyirady, 2016).
Pathophysiology / Biochemical Mechanisms
Pathophysiology
Amyloid Light Chains
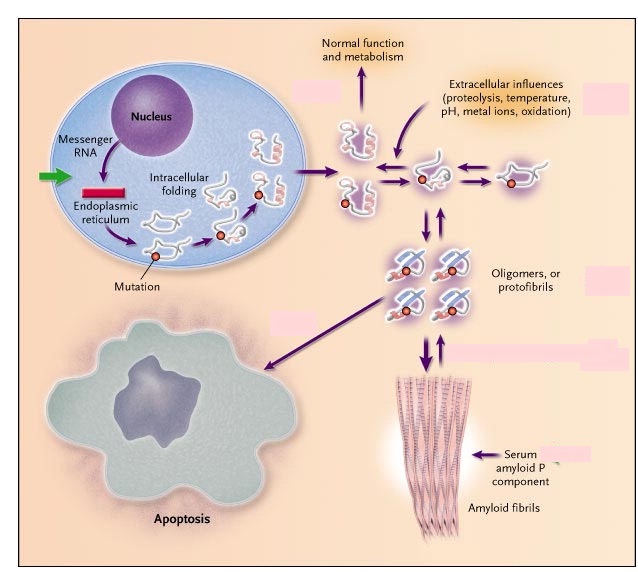
Amyloid Light-chain (AL) amyloidosis is a bone marrow disorder, specifically a plasma cell dyscrasia, which forms misfolded immunoglobulin light chains that accumulate in large quantities and deposit in the extracellular matrix of many organs, rendering them dysfunctional (Zhang, Huang & Li, 2017).
Amyloidogenic Immunoglobulin light chains are produced by abnormally, over-proliferative monoclonal populations of plasma cells that mass-produce antibodies like immunoglobulin(Ig)(Ramirez-Alvarado, 2012, Zhang, Huang & Li, 2017). Once in blood circulation, these Ig light chains unfold, bind to other light chains and extracellular matrix (ECM) components and deposit as insoluble amyloid fibrils in tissues and organs (Ramirez-Alvarado, 2012).
http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html
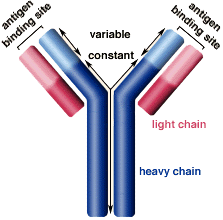
Figure 3: Structure of the Immunoglobulin antibody
The Ig antibodies produced by plasma cells are composed of two light chains, classified as kappa and lambda, and two heavy chains linked via sulfide bonds (Ramirez-Alvarado, 2012). The tendency of Ig light chains to develop fibrillogenesis depends on the primary structure of the light chains (Zhang, Huang & Li, 2017). The ratio of the two light chain classes, kappa and lambda, in normal adults is approximately 2:1 however, in AL patients, the lambda light chains are seen four times as much compared to kappa (Zhang, Huang & Li, 2017, Weiss et al., 2016). In the Immunoglobulin light chains, the variable regions are highly mutated relative to the closest germline genes (Perfetti et al., 1998).
http://http://www.nejm.org/doi/full/10.1056/NEJMra023144
Figure 4: ECM components and Ig light chains combine to form a β-super-secondary structure (Merlini & Belloti, 2003). These β- sheet polypeptides form a protofilament which then wraps around other protofilaments to shape the amyloid fibrils (Merlini & Belloti, 2003).
The amyloid precursors form and are secreted as native proteins, however, they escape the intracellular quality control system where the mutant light chains are not recognized (Merlini & Bellotti, 2003). Once in the ECM, these light chains unfold and reach an equilibrium between fully folded and partially folded state (Merlini & Bellotti, 2003). Local factors such as low pH, oxidative stress, high temperature and limited proteolysis shift this equilibrium towards the partially folded state, forming the protofibrils (Merlini & Bellotti, 2003). The protofibrils then go onto forming amyloid fibrils and they also cause cellular toxicity by inducing apoptosis in target tissue cells (Merlini & Bellotti, 2003).
Factors Influencing Fibrillogenesis
Plasma Cells
- Malignant plasma cells can be identified by low levels of glycoproteins CD19, CD27, CD38, CD45 and high levels of CD28, CD 33, CD56 compared to normal plasma cells (Zhang, Huang & Li, 2017, Paiva et al., 2010).
- In a study conducted on 17 AL amyloidosis patients, 81% had cytogenetic abnormalities such as translocation of chromosome 14q32, monosomy 13, trisomies 9, 15, 11 and 3 (Zhang, Huang & Li, 2017 Warsame et al., 2015).
Post Translational Modifications
- Glycosylation may promote amyloid fibrillogenesis by altering protein solvation energy, increasing binding strength at certain sites and protecting some regions from degradation by proteases (Zhang, Huang & Li, 2017, Petukhov, Cregut, Soares, & Serrano, 1999; Schachter, 1984; Wallick, Kabat, & Morrison, 1988).
Extracellular Matrix Components
- Serum Amyloid P protects the amyloid fibrils from proteolytic degradation (Zhang, Huang & Li, 2017, Tennent, Lovat & Pepys, 1995)
- Heparan Sulfate proteoglycans(HSPG) interact with ligands such as growth factors, cytokines, enzymes, etc (Zhang, Huang & Li, 2017). They induce precursors to take-up amyloidogenic conformations and also promote protofilament assembly into mature fibrils through stabilization (Zhang, Huang & Li, 2017, McLaurin, Franklin, Zhang, Deng, & Fraser, 1999; Ren et al., 2010)
Extracellular Chaperones (ECs)
- ECs have a controversial role in AL amyloidosis (Zhang, Huang & Li, 2017). Some studies show that alpha2-macroglobulins ( a type of EC) facilitate degradation of beta-amyloid by receptor-mediated endocytosis and degradation by lysosome with the help of clusterin (EC) (Zhang, Huang & Li, 2017, Narita, Holtzman, Schwartz, & Bu, 1997). However, other studies have shown that ECs bind to amyloid fibrils and protect them from degradation by proteases (Zhang, Huang & Li, 2017).
Prevention
As far as discernable, the state of the current research suggests that no direct preventive measures exist for AL Amyloidosis. However, there are preventive measures for similar diseases in the same family. According to Ghiso and Frangione (2002), one such disease is Alzheimer’s, which is the most prevalent form of amyloidosis in humans. The mechanisms of the disease are similar to AL Amyloidosis in the sense that amyloid deposits form in the brain parenchyma of patients with Alzheimer’s disease (Ghiso & Frangione, 2002).
In the case of Alzheimer’s disease, the transport protein Transthyretin prevents amyloid formation (Schwarzman et al., 1994). Alzheimer’s disease is part of the same family as AL amyloidosis, with the family being systemic amyloidosis. Similarly, colchicine prevents amyloidosis of familial Mediterranean fever (Zemer et al., 1986). This fever is also within the family of systemic amyloidosis like AL amyloidosis. Colchicine is a drug that is administered to the individual to help combat the effects of the amyloid deposits.
Treatment / Management
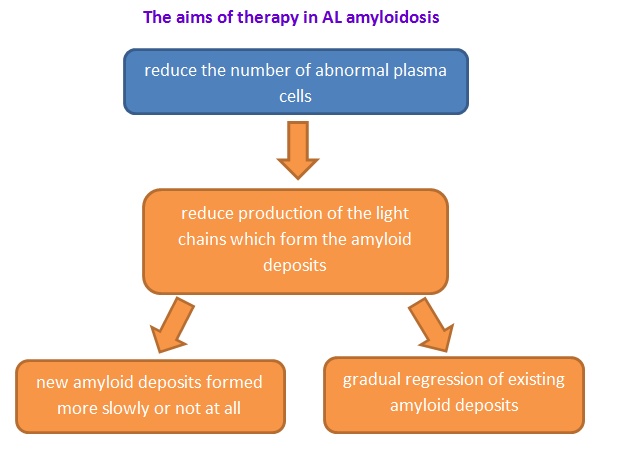
Recently, limited treatment is available that specifically targets the amyloid and most of them are supportive measure (Ma, G., & Ra, K., 1994; Tidy, C., 2014) In order to treat this disease, the aim is to suppress the development of the amyloid-forming protein and manage the symptoms. (Tidy, C., 2014)Therefore, people may use different treatments depends on what type of amyloidosis and the stabilization of organs (Papadakis, M.A., & McPhee, S. H., 2017).
http://www.amyloidosis.org.uk/al-amyloidosis/treatment/
Figure 6: The aims of therapy in AL amyloidosis.(Wechalekar, A., 2017).
Chemotherapy and Autologous Stem-Cell Transplantation:
High-dose chemotherapy medicines (such as melphalan) with autologous stem cell transplant is the current treatment for patients with AL Amyloidosis.(Derrer, T.D., 2015; NHS choices, 2017; Shiel, C.W., & Balentine, R. J., 2017) Since AL Amyloidosis is deposition of abnormal proteins in tissues and organs of the human body leading to organ dysfunction (i.e. heart, liver, kidney etc.) (Papadakis, M.A., & McPhee, S. H., 2017). By using this treatment, the aim is to reduce the light chain protein production (the substance that can form amyloid) and deposition which can cause AL Amyloidosis. On the other hand, this treatment can arrest further progression organ dysfunction (Papadakis, M.A., & McPhee, S. H., 2017). Using high doses of chemotherapy medicines like melphalan, a high rate of remission was observed (Child et al., 2003).This treatment is followed by autologous stem cell transplantation, however, some patients are unsuitable and cannot endure its toxicity leading to febrile neutropenia, mucositis, delirium and atrial dysrhythmias (Bernard et al., 2015). In the case of Renal failure, patients may require dialysis and diuretic medications to remove excess water from their body (Tidy, C., 2014; Derrer, T.D., 2015). If people cannot undergo the treatment properly, they may use oral melphalan and dexamethasone (Derrer, T.D., 2015; NHS choices, 2017; Shiel, C.W., & Balentine, R. J., 2017). These two chemotherapy medicines can give good hematological and organ response(Mahmood et al, 2014). In the case of chemotherapy, some commonly used medications are melphalan, cyclophosphamide, dexamethasone, lanalidomide and bortezomib (Papadakis, M.A., & McPhee, S. H., 2017). However, chemotherapy treatment has a related mortality rate of approximately 8% in patients with AL Amyloidosis, because their organs fail to recover even after therapy. However, since the treatment facilitates amyloid dissolution and corrects the amyloid protein folding, many patients have successful recovery (Papadakis, M.A., & McPhee, S. H., 2017).
Anti-Inflammatory Medicines:
Secondary systemic amyloidosis (AA) usually presents itself with some inflammation. Therefore, physicians normally treat it with chemotherapy and anti-inflammatory medicines which are basically steroids (Derrer, T.D., 2015; NHS choices, 2017, Papadakis, M.A., & McPhee, S. H., 2017). Using steroids sometimes results in a bad reaction due to the side effects of chemotherapy (NHS choices, 2017), and so patients must be regularly monitored to prevent such reaction. On the other hand, secondary amyloidosis may co-exist with another illness such as a chronic infection (i.e. Tuberculosis and osteomyelitis) and chronic inflammatory disease (i.e. Rheumatoid arthritis). For Rheumatoid arthritis, physicians usually treat it by using Chlorambucil (Derrer, T.D., 2015).
Organ Transplant:
http://www.mayoclinic.org/tests-procedures/liver-transplant/details/what-you-can-expect/rec-20211848
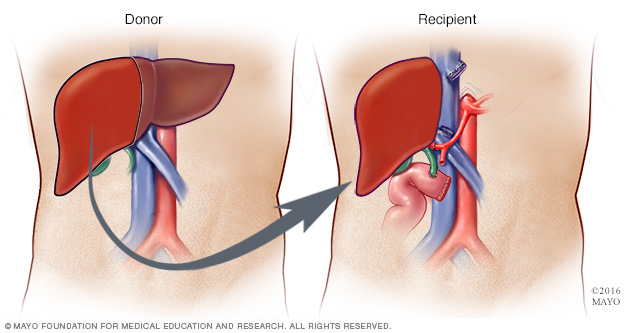
Figure 7: The Liver Transplant (Mayo, 2017).
Liver transplant is also one of the treatment options in case of liver failure from AL Amyloidosis (Derrer, T.D., 2015). Also, some patients may have hereditary amyloidosis or ATTR (Derrer, T.D., 2015). In this disease, the liver produces mutated transthyretin which forms amyloid deposits (Papadakis, M.A., & McPhee, S. H., 2017). In order to arrest this process, physicians will strongly suggest the patients do a liver transplant ( NHS choices, 2017). However, they need to also consider the rejection by the human body.
From the information provided above, it can be deduced that depending on the condition of amyloidosis, the amyloid protein may affect some vital organs such as kidney and heart. If the vital organs get an infection, an organ transplant will become a reasonable treatment option (i.e. kidney or heart transplant) (Derrer, T.D., 2015; NHS choices, 2017).
Colchicine:
For Familial Mediterranean fever, means 2nd amyloidosis in inflammatory bowel disease, physicians will suggest to use Colchicine (Shiel, C.W., and Balentine, R. J., 2017).
Other Treatments:
Amyloidosis can occur under different conditions, affecting different vital organs. People may have swelling of the tongue, therefore, they can add some thickeners to the fluids in order to prevent choking (Derrer, T.D., 2015). They may also have swelling in their legs and feet and so they may use compression stockings which help relieve swelling (Derrer, T.D., 2015). Lastly, f people have gastrointestinal amyloidosis, they can modify their diet in order to prevent the disease progression (Derrer, T.D., 2015).
Social Implications
AL Amyloidosis or Amyloidosis in general is a disease not very known by the majority of society. Among those who do know of the disease, there are often a lot of misconceptions or incorrect understanding of the scientific information. However, a lot is being done by corporations and foundations in order to spread correct information about the disease to the general public in order to create awareness.
The Amyloidosis Foundation is one such foundation. According to their website, they were created by the merging of the Amyloidosis Research Foundation and the Amyloidosis Support Network in 2007 (“Us - Amyloidosis Foundation”, n.d.). Some of the goals of the foundation is that they are looking to create awareness, collect donation for patients, and invest in research that will go a long way in helping those affected by the disease. As can be seen on the website, they also have many inspirational stories of former patients who have gone through having the AL Amyloidosis disease (“Us - Amyloidosis Foundation”, n.d.). This is a fantastic resource for patients that have recently discovered they have AL Amyloidosis as they may feel they are all alone. However, on the contrary, this is not the case and in fact through reading the stories on the website, they can see there are many other people in the same situation as them. Thus, the foundation's stories of other patients acts as a sort of moral encouragement for new patients. Finally, the foundation also provides updates on the latest news regarding AL Amyloidosis research, among other things. This too acts as a great resource for patients as not all of them will have the technical knowledge to read the actual scientific research articles. Instead, they can read an adapted and summarized version presented on the foundation's news website. This still allows them to feel in the know about the current state of research into discovering a cure for this devastating disease. Overall, the foundation acts as a bridge to connect patients with resources to help them better overcome their disease and to live a happier, fuller life.
Further Research / Applications
There is still a lot of research that needs to be done on AL Amyloidosis in order to successfully diagnose and treat everyone who gets diagnosed. The research being conducted spans a variety of platforms such as alternate treatments, enhancements to current treatments, earlier diagnosis methods and prevention methods. The focus is on discovering tools through which diagnosis can be made earlier, even before the appearance of symptoms, in order to provide treatment earlier and to help increase success and survivor-ship.
Epigallocatechin-3-Gallate (EGCG):
Epigallocatechin-3-Gallate (EGCG) is being looked at as a potential treatment for AL Amyloidosis. EGCC is the most abundant catechin in tea. EGCC is studied greatly today for its potential effects on human health and diseases. It is used in many dietary supplements. It is found in high concentrations in the dried leaves of green tea, white tea and black tea (Andrich et al., 2016). Trace amounts are found in foods such as apple skins, plums, onions and pecans (Andrich et al., 2016). EGCC is currently the subject of pre-clinical studies involving autoimmune diseases, inflammation and rheumatoid arthritis (Andrich et al., 2016). Recently, EGCC is being looked at as a secondary treatment for AL Amyloidosis. Research so far has suggested that EGCG specifically inhibits the second aggregation phase causing the formation of more stable non-amyloid light chain aggregates (Andrich et al., 2016). Scientists are most intrigued by the fact that EGCG intervention does not depend on the individual sequences of the light chains and thus can potentially be universally used as a treatment (Andrich et al., 2016). Further research is required in order to determine if EGCG would work at larger quantities and if it sustains inhibition for a prolonged period of time.
http://toptestosteronesupplements.com/epigallocatechin-gallate-egcg-ingredient-breakdown/
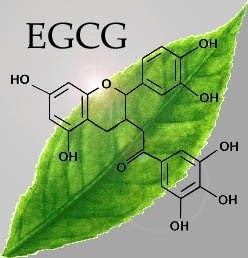
Figure 8: Molecular structure of EGCG. EGCG found commonly in a variety of dried tea leaves. Looked at as a potential treatment for AL Amyloidosis.
Monoclonal Antibodies - NEOD001:
The most cutting-edge approach to AL Amyloidosis is the use of monoclonal antibodies which would clear the amyloid from the affected organs. Recent studies looking at NEOD001 have been very promising. NEOD001 is a monoclonal antibody that specifically targets and eliminates the aggregated amyloid that accumulates in AL patients(Gertz et al., 2016). In one study, 69 patients were administrated NEOD001 post-chemotherapy (Gertz et al., 2016). Response rate was 53% in cardiac patients and 64% among renal patients with little to no adverse side-effects (Gertz et al., 2016). NEOD001 also demonstrated improved neuropathy (Gertz et al., 2016). There are high hopes for this treatment and it is currently in phase two of pre-clinical trials with phases 3 study planned.
Genetically Engineered Cells:
Pre-Clinical testing is on-going on a potential treatment for AL Amyloidosis where a patient’s own immune system is genetically engineered to recognize and kill abnormal blood cells when reintroduced into the body (Rosenzweig, M., 2017). The current goal of the research is to evaluate blood cells of patients with AL Amyloidosis and to find a specific protein that could be targeted (Rosenzweig, M., 2017). This protein must be unique to the infected blood cells of AL Amyloidosis patients. Once this key step is completed, in-laboratory testing of genetically engineered cells will occur.
Study of Gene Expression Changes/Mutations:
To date, mutations/proteins involved in AL Amyloidosis are poorly understood and the lack of knowledge is holding back scientists from studying a broader range of treatments as well as being able to diagnose patients much earlier in life. A large portion of scientific research on AL Amyloidosis is spent on understanding and locating varies proteins, mutations and gene expressions that play a role in the development of this disease (Zhou et al., 2012). Identifying and understanding such aspects not only helps with further treatment development but also helps diagnosis patients early in the disease stage or ideally before symptoms even occur. A large-scale characterization of the mutations and gene expression changes that occur when developing AL Amyloidosis is currently occurring (Zhou et al., 2012).
Clear Amyloid Protein Deposits (Immunotherapy / Physical):
Clearing Amyloid protein deposits directly was previously seen as impossible but recent research has now proved this misconception incorrect. It has been discovered that immune cells known as Scavenger Cells, have the capabilities of engulfing and breaking apart Amyloid deposits (Rosenzweig et al., 2017). However, the production and effectiveness of such cells are not at the levels required to clear large deposits of amyloids (Rosenzweig et al., 2017). New immunotherapy treatments are trying to accelerate the production and functionality of such immune cells in order to be able to clear larger deposits as well as clear them quickly (Rosenzweig et al., 2017). Three of such immuno-therapies are currently in stage 3 of clinical trials. There is a lot of hope in this form of treatment due to it being both efficient, effective and safe (Rosenzweig et al., 2017).
Basic research has also begun on constructing machinery that can physically be inserted into the body, target amyloid deposits, break the amyloid deposits into smaller piece and finally remove all traces of the amyloid deposits (Rosenzweig et al., 2017). Constructing such machinery while ensuring the patient's safety is not an easy task. Research into such machinery has only recently begun and is far away from being a realistic treatment method for AL Amyloidosis.
Proteasome Inhibitors:
There are currently 2 drugs in clinical trials that specifically target proteasomes required in the formation of the amyloid aggregates (Sanchorawala et al., 2017). Inhibition of such prevents such proteasomes from doing their role of degrading specific proteins (Sanchorawala et al., 2017). The proteins they normally degrade are proteins that stop the aggregation and formation of amyloid fibrils. Thus the 2 drugs would keep the preventive proteins intact and amyloid fibrils would be prevented from every forming (Sanchorawala et al., 2017). The two drugs carfilzomib and ixazomib are very close to market release.
Other:
There are several other viable treatments being researched today in hopes of finding better treatments for AL Amyloidosis. A few others include:
1. Antibiotics: New antibiotics that interfere with Amyloid deposit formation. Does not stop poor light-chain formation but prevents the formation of dangerous and large Amyloid deposits (Gertz et al., 2016).
2. Targeting of SAP Protein: Potential new drug treatments are targeting the SAP protein which is a very specific protein structure found exclusively on Amyloid deposits and no other human cells/structures. Targeting such a protein will prevent any harm being done on normal healthy cells (Wechaleka et al., 2016).
3. Vaccines: The formation of a new vaccine is being studied that when administrated would protect vital organs such as the heart from Amyloid Formation. The basic idea is that such antibodies would coat and organs and prevent amyloid fibrils from attaching and growing (Sanchorawala et al., 2017).
Conclusion
Ultimately, AL Amyloidosis is a dangerous bone marrow disorder that due to abnormal antibody light-chains forms lethal amyloids that disrupt the health of organs (Nienhuis, Bijzet & Hazenberg, 2016). AL Amyloidosis is still rather lethal even with newer treatments. It is important for older individuals to be aware, educated and take initiative. The Amyloidosis Foundation website is a great source for more information - provides background info, charities/fundraiser info, potential grants and future planned research. Researchers believe AL Amyloidosis will no longer be a threat in 10 years due to the current success rate of research and the release of more treatments.
References
- Amyloidosis. (n.d.). Cedars-sinai.edu. Retrieved 4 October 2017, from https://www.cedars-sinai.edu/Patients/Health-Conditions/Amyloidosis.aspx
- Amyloidosis Foundation. (n.d.). Retrieved from http://www.amyloidosis.org/
- Andrich, K., Hegenbart, U., Kimmich, C., Kedia, N., Bergen III, C.R., Schonland, S., Wanker, E.E., & Bieschke, J. (2016). Aggregation of full length immunoglobulin light chains from AL Amyloidosis patients is remodeled by epigallocatechin-3-gallate. Journal of Biological Chemistry, 292, 2328-2344. https://doi.org/10.1074/jbc.M116.750323
- BECKERMAN, M. (2015). FUNDAMENTALS OF NEURODEGENERATION AND PROTEIN MISFOLDING DISORDERS (p. 5). [S.l.]: SPRINGER INTERNATIONAL PU.
- Bernard, R., Chodirker, L., Masih-khan, E., Jiang, H., Franke, N, Kukreti, v., . . .Chen, C. (2015). Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplantation, 50, 95-99. https://doi.org/10.1038/bmt.2014.226
- Biology.arizona.edu. (2017). Antibody Structure. [online] Available at: http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html [Accessed 6 Oct. 2017].
- Child, J.A., Morgan, J.G., Davies, E.F., Owen, R.G., Bell, S.E., Hawkins, K., Brown, J., Drayson, M.T., & Selby, P.J. (2003). High-Dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med, 348, 1875-1883. https://doi.org/10.1056/NEJMoa022340
- Derrer, T.D. (2015). Amyloidosis. WebMD. Retrieved from: http://www.webmd.com/cancer/lymphoma/amyloidosis-symptoms-causes-treatments#1
- Desport, E., Bridoux, F., Sirac,C., Delbes,S., Bender, S., Fernandez, B., … Jaccard, A. (2012). AL Amyloidosis. Orphanet Journal of Rare Diseases, 7, 54. https://doi.org/10.1186/1750-1172-7-54
- Ebert, E. C., & Nagar, M. (2008). Gastrointestinal manifestations of amyloidosis. The American Journal of Gastroenterology. 103(3), 776–787. https://doi.org/10.1111/j.1572-0241.2007.01669.x
- Gandelman, G. (2012). Cardiac Amyloidosis. The New York Times. Retrieved from: http://www.nytimes.com/health/guides/disease/cardiac-amyloidosis/overview.html?mcubz=0
- Gertz, M.A., Landau, H., Comenzo, R.L., Seldin, D., Weiss, B., & Zonder, J. (2016). First-in-Human Phase I/II Study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. Journal of Clinical Oncology, 34, 1097-1103. https://doi.org/10.1200/JCO.2015.63.6530
- Ghiso, J., & Frangione, B. (2002). Amyloidosis and Alzheimer’s disease. Advanced drug delivery reviews, 54(12), 1539-1551.
- Jantunen, E., Kuittinen, T., Penttilä, K., Lehtonen, P., Mahlamäki, E., & Nousiainen, T. (2006). High-dose melphalan (200 mg/m2) supported by autologous stem cell transplantation is safe and effective in elderly (greater than or equal to65 years) myeloma patients: comparison with younger patients treated on the same protocol. Bone Marrow Transplantation, 37, 917-922. https://doi.org/doi:10.1038/sj.bmt.1705360
- Kyle, R. (2001). Amyloidosis: a convoluted story. British Journal Of Haematology, 114(3), 529-538. http://dx.doi.org/10.1046/j.1365-2141.2001.02999.x
- Liu, P.P., & Smyth, D. (2015). Wild-Type transthyretin amyloid cardiomyopathy a missed cause of heart failure with preserved ejection fraction with evolving treatment implications. Circulation,133,245-247. https://doi.org/10.1161/CIRCULATIONAHA.115.020351
- Ma, G., & Ra, K. (1994). Amyloidosis: prognosis and treatment. Semin Arthitis Rheum., 24(2), 124-138. https://doi.org/10.1016/S0049-0172(05)80006-X
- McLaurin, J., Franklin, T., Zhang, X., Deng, J., & Fraser, P. E. (1999). Interactions of Alzheimer amyloid-beta peptides with glycosaminoglycans effects on fibril nucleation and growth. European Journal of Biochemistry, 266(3), 1101–1110. https://doi.org/10.1046/j.1432-1327.1999.00957.x
- Merlini, G., & Bellotti, V. (2003). Molecular Mechanisms of Amyloidosis. New England Journal of Medicine, 349(6), 583–596. https://doi.org/10.1056/NEJMra023144
- Narita, M., Holtzman, D. M., Schwartz, A. L., & Bu, G. (1997). Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. Journal of Neurochemistry, 69(5), 1904–1911. https://doi.org/10.1046/j.1471-4159.1997.69051904.x
- Ngan, V. (2015). Purpura. DermNet New Zealand. Retrieved from https://www.dermnetnz.org/topics/purpura/
- NHS choices. (2017). Amyloidosis. NHS choices. Retrieved from: http://www.nhs.uk/Conditions/amyloidosis/Pages/Introduction.aspx#treatment
- Nienhuis H, L., A, Bijzet J., & Hazenberg B, P, C. (2016). The prevalence and management of systemic amyloidosis in western countries. Kidney Dis, 2, 10-19. https://doi.org/10.1159/000444206
- Nyirady, J. (2016). Primary systemic amyloidosis. MedScape. Retrieved from http://emedicine.medscape.com/article/1093258-overview
- Paiva, B., Almeida, J., Pérez-Andrés, M., Mateo, G., López, A., Rasillo, A., Orfao, A. (2010). Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry. Part B, Clinical Cytometry, 78(4), 239–252. https://doi.org/10.1002/cyto.b.20512
- Palladini, G., Perfetti, V., Obici, L., Caccialanza, R., Semino, A., Adami, F.,Cavallero, G., Rustichelli, R., Virga, G., & Merlini, G. (2004). Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood, 103, 2936-2938. https://doi.org/10.1182/blood-2003-08-2788
- Papadakis, M.A., & McPhee, S.J. (2017) Current: medical diagnosis & treatment. USA: McGraw-Hill Education
- Perfetti, V., Ubbiali, P., Vignarelli, M. C., Diegoli, M., Fasani, R., Stoppini, M.,Merlini, G. (1998). Evidence that amyloidogenic light chains undergo antigen-driven selection. Blood, 91(8), 2948–2954. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9531605
- Petukhov, M., Cregut, D., Soares, C. M., & Serrano, L. (1999). Local water bridges and protein conformational stability. Protein Science: A Publication of the Protein Society, 8(10), 1982–1989. https://doi.org/10.1110/ps.8.10.1982
- Ramirez-Alvarado, M. (2012). Amyloid formation in light chain amyloidosis. Current Topics in Medicinal Chemistry, 12(22), 2523–2533. https://doi.org/10.2174/1568026611212220007
- Real de Asúa, D., Costa, R., Galván, J. M., Filigheddu, M. T., Trujillo, D., & Cadiñanos, J. (2014). Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clinical Epidemiology, 6, 369–377. http://doi.org/10.2147/CLEP.S39981
- Ren, R., Hong, Z., Gong, H., Laporte, K., Skinner, M., Seldin, D. C., … Trinkaus-Randall, V. (2010). Role of glycosaminoglycan sulfation in the formation of immunoglobulin light chain amyloid oligomers and fibrils. The Journal of Biological Chemistry, 285(48), 37672–37682. https://doi.org/10.1074/jbc.M110.149575
- Rosenzweig, M., Urak R., Walter, M., Lim, L., Sanchez, J.F., Krishnan, A., Forman, S., & Wang, X. (2017). Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis. Cytotherapy, 19(7), 861-866. https://doi.org/10.1016/j.jcyt.2017.03.077
- Rosenzweig, M., Urak, R., Walter, M., Lim, L., Sanchez, J.F., Krishnan, A., Forman, S., & Wang, X. (2017). Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis. Cytotherapy, 19, 861-866. https://doi.org/10.1016/j.jcyt.2017.03.077
- Sanchorawala, V. (2006). Light-Chain (AL) Amyloidosis: diagnosis and treatment. Clinical Journal Of The American Society Of Nephrology, 1(6), 1331-1341. http://dx.doi.org/10.2215/cjn.02740806
- Sanchorawala, V., Palladini, G., Kukreti, V., Zonder, J.A., Cohen, A.D., Seldin, D.C., . . . Merlini, G. (2017). A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory light-chain (AL) amyloidosis. Blood, 130, 597-605. https://doi.org/10.1182/blood-2017-03-771220
- Schachter, H. (1984). Glycoproteins: their structure, biosynthesis and possible clinical implications. Clinical Biochemistry, 17(1), 3–14. https://doi.org/10.1016/S0009-9120(84)90360-6
- Schwarzman, A., Gregori, L., Vitek, M., Lyubski, S., Strittmatter, W., & Enghilde, J. et al. (1994). Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proceedings Of The National Academy Of Sciences, 91(18), 8368-8372. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC44607/
- Shiel, C.W., & Balentine, R. J. (2017). Amyloidosis. MedicineNet. Retrieved from: http://www.medicinenet.com/amyloidosis/article.htm#what_is_the_treatment_for_amyloidosis
- Sipe, J. (2008). Amyloid Proteins. The Beta Sheet Conformation and Disease. (1st ed., p. 4). Weinheim: Wiley-VCH.
- Tennent, G. A., Lovat, L. B., & Pepys, M. B. (1995). Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proceedings of the National Academy of Sciences of the United States of America, 92(10), 4299–4303. Received from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC41931/pdf/pnas01486-0235.pdf
- Tidy, C. (2014). Amyloidosis. Patient. Retrieved from: https://patient.info/doctor/amyloidosis-pro
- Us - Amyloidosis Foundation. Amyloidosis Foundation. Retrieved 7 October 2017, from http://www.amyloidosis.org/us/#our-history
- Wallick, S. C., Kabat, E. A., & Morrison, S. L. (1988). Glycosylation of a VH residue of a monoclonal antibody against alpha (1-6) dextran increases its affinity for antigen. The Journal of Experimental Medicine, 168(3), 1099–1109. . https://doi.org/10.1084/jem.168.3.1099
- Warsame, R., Kumar, S. K., Gertz, M. A., Lacy, M. Q., Buadi, F. K., Hayman, S. R., … Dispenzieri, A. (2015). Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer Journal, 5, 310. https://doi.org/10.1038/bcj.2015.34
- Wechalekar, A. (2017). Treatment of AL Amyloidosis. NAC. Retrieved from: http://www.amyloidosis.org.uk/al-amyloidosis/treatment/
- Wechalekar, A.D., Gilmore, J.D., & Hawkins P.N. (2016). Systemic amyloidosis. The Lancet, 387(38), 2641-2654. https://doi.org/10.1016/S0140-6736(15)01274-X
- Weiss, B. M., Wong, S. W., & Comenzo, R. L. (2016). Beyond the plasma cell: emerging therapies for immunoglobulin light chain amyloidosis. Blood, 127(19), 2275–2280. https://doi.org/10.1182/blood-2015-11-681650.
- Zemer, D., Pras, M., Sohar, E., Modan, M., Cabili, S., & Gafni, J. (1986). Colchicine in the prevention and treatment of the amyloidosis of familial mediterranean fever. New England Journal Of Medicine, 314(16), 1001-1005. http://dx.doi.org/10.1056/nejm198604173141601
- Zhang, C., Huang, X., & Li, J. (2017a). Light chain amyloidosis: Where are the light chains from and how they play their pathogenic role?. Blood Reviews, 31(4), 261–270. https://doi.org/10.1016/j.blre.2017.03.002
- Zhou, P., Hoffman, J., Landau, H., Hassoun, H., Lyer, L., & Comenzo, R.L. (2012). Clonal Plasma Cell Pathophysiology and clinical features of disease Are linked to clonal plasma cell Expression of cyclin D1 in systemic light-chain amyloidosis. Clinical Lymphoma Myeloma and Leukemia, 12, 49-58. https://doi.org/10.1016/j.clml.2011.09.217