Table of Contents
Immunology
What is Immunology?
Immunology is a branch in science that focuses on studying the body’s immune system. The immune system incorporates a variety of cellular factors and mechanisms that function to protect the human body from infections and diseases. However, malfunctions can occur within the immune system leading to disease, disorders, allergies, cancer, and even death. (British Society for Immunology, n.d.)
How Does the Immune System Work?
Mammals are said to have some of the most sophisticated immune systems in the animal kingdom. Humans and other mammals are consistently exposed to pathogenic and organisms that can threaten the body’s homeostasis. Therefore, the immune system has evolved to have complex protective mechanisms to control toxic organisms in the body (Chaplin, 2010). For most vertebrates, the immune system has two main functions. First, the function of the immune system is to detect pathogens and separate them from the body’s natural, healthy tissue (Beck & Habicht, 1996). It is also essential to regulate non-pathogenic organisms that are beneficial to the body. Second, the immune system must eliminate the pathogens, often bacteria and viruses, without damaging its own tissue. Furthermore, it also has the ability to get rid of dead or injured cells from the body that have been damaged by injury or infectious disease (Chaplin, 2010).
Immune Cells and Response
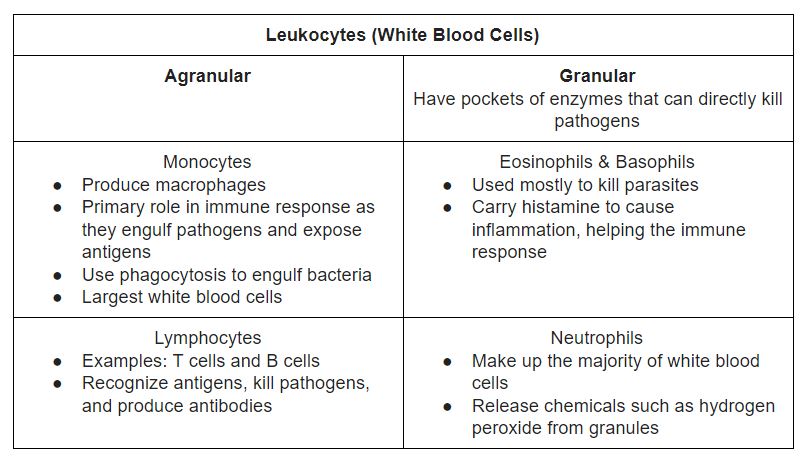
An intact immune response includes contributions from many subsets of leukocytes, or white blood cells, which are formed in the bone marrow. Leukocytes have many different cell types that have interdependent but specialized functions, collectively preventing infection (Chaplin, 2010). Their functions are outlined in Table 1 below.
Table 1. Summary of white blood cell types and functions (Brain, 2019).
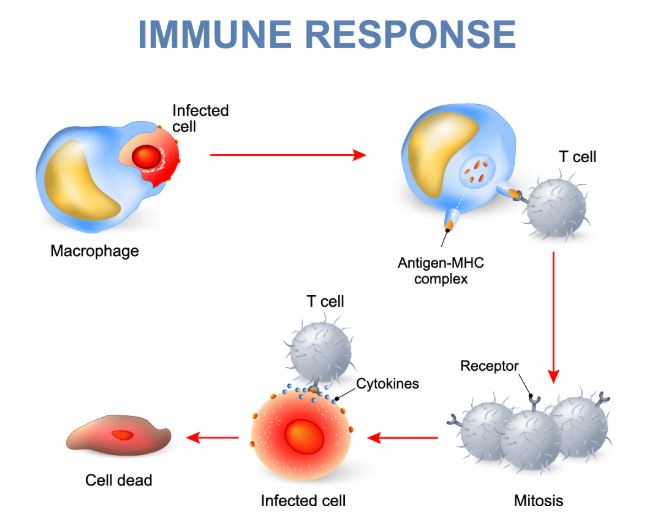
Macrophages are the most important cells involved in starting the immune response. These cells pick up foreign microorganisms or other infected cells and allow their antigens, or cell markers, to be detected by lymphocytes. Stimulated macrophages also engulf bacteria through a process called phagocytosis. Once activated, macrophages release cytokines, which are signal molecules, such as interleukin-1 (IL1) and interleukin-6 (IL6) to recruit lymphocytes (Linnemeyer, 2008). Other cells are used to kill parasites and bacteria directly and allow for the excretion of these dead particles. The immune response can occur in all organs where white blood cells are present, but this is primarily in the lymph nodes and the spleen. Other organs include the tonsils, the lungs, and the skin. The immune response begins when blood and fluid is passed through these organs (NIAID, 2019). This process is outlined in Figure 1 and the types of immunity are discussed below, which utilize these processes in more detail.
Figure 1. Common immune response in humans involving macrophage and T-cell recruitment (Immuneresponse.org, n.d).
Evolution of the Immune Response
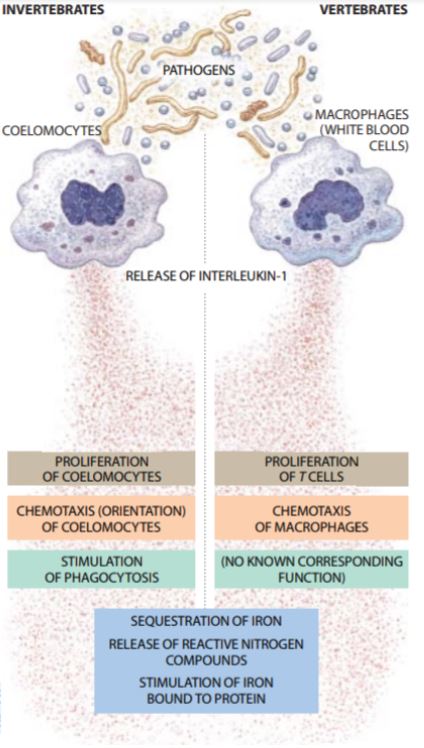
Studies have shown that the immune system in vertebrates had evolved to conserve host defense mechanisms of early invertebrates while developing more advances mechanisms and signalling pathways. Early work done by Beck & Habicht (1996) showed that isolating various proteins from invertebrates resemble cytokines involved in vertebrate immune responses. The researches isolated fluid from the Atlantic starfish species and found proteins similar in function, finding that IL-1 and IL-6 function similarly to invertebrate proteins involved in immune responses. They also found that human cytokines are analogous to molecules called coelomocytes in worms. In addition, cytokines involved in invertebrate immunity, known as coelomocytes, function the same way in vertebrates, as shown in Figure 2. These cells produced IL-1 and stimulate macrophages as they do in humans (Beck & Habicht, 1996).
Figure 2. Comparison of invertebrate and vertebrate immune response (Beck & Habicht, 1996).
Disorders of the Immune System
Immunodeficiency disorders result when the immune system response is less active or is not functioning optimally. This can result in life threatening or chronic conditions, resulting from genetic disorders such as severe combined immunodeficiency, acquired conditions such as HIV/AIDS, or the use of medication to suppress the immune system. In contrast, autoimmune disorders result from a hyperactive immune system that attack the body’s healthy tissue as if they were foreign. Examples of autoimmune disease include type 1 diabetes and rheumatoid arthritis (O’Byrne & Dalgleish, 2001).
The Immune System: Types and Mechanisms
Naturally, the immune system is built up of many different cells and proteins that function to protect the body against pathogens and foreign invaders. The immune system can be broken down into two sections, which are often referred to as the ‘lines of defense’. The two lines of defence are; innate immunity and adaptive immunity (Hoebe et al., 2004).
Innate Immunity
This type of immunity is the bodies first line of defense, it is also known as antigen-independent or non-specific immunity. Antigens are substances that can induce an immune response, which are usually made up of proteins, polysaccharides, lipids or nucleic acids. So when immunity is antigen-independent, this mean that there is no requirement for the body to have previous exposure to that pathogen or invader. The mechanism occurs immediately or within hours after encountering the antigen. There are four main ways in which innate immunity provides a defensive barrier to the body (Medzhito & Janeway, 2000).
Anatomical: The skin and mucous membrane acts as an immediate barrier.
Physiological: Certain homeostatic mechanisms that exist prevent the invasion of pathogens such as body temperature as well as pH levels.
Endocytic & Phagocytic: Cells that are capable of taking up or removing particles contribute to protection.
Inflammation: Cells that evoke an inflammation response to gather necessary immune cells are vital for innate immunity.
The function of Innate immunity is to recruit necessary immune cells to the sites of infection and inflammation via cytokines. Cytokines are activation signals which mediate and regulate immunity, they are secreted by specific immune cells and have an effect on others (Dinarello, 2000). When cytokines are produced, this leads to a release of various molecules such as antibodies and proteins that function to identify foreign invaders (antigen) and preparing them for phagocytosis. Phagocytosis is referred to as ‘cell eating’ and it involves the engulfment of microbes to help eradicate unwanted cell debris (Stossel, 1974). It is important to discuss the relationship between antibodies and antigens, as this is a key concept of how our body develops immunity.
Antigen: A substance that can induce an immune response (Sela-Culang et al., 2013).
Antibody: These are proteins that recognize and bind to antigens. They are produced by B-cells in response to antigen exposure (Sela-Culang et al., 2013).
There are many cells that are involved in the innate immune response, and they each play a different role within the process. Key players in this mechanism are phagocytes, which are types of cells that are capable of engulfing and absorbing bacteria as previously mentioned.
Phagocytes can be broken down into two types; neutrophils and macrophages. Both types have cell engulfment properties, however, neutrophils produce granules that assist in eliminating pathogenic microbes and macrophages are involved in antigen presentation with T-cells . Other cells that play a role in antigen presentation are dendritic cells, they also act as messengers between innate and adaptive immunity (Warrington et al., 2014).
Adaptive Immunity
The next line of defense that our body utilizes is called adaptive immunity, and this develops when the first line is compromised. Meaning, when the immune system is unsuccessful in eliminating pathogens and an infection is persistent. The function of the adaptive or specific immunity system is to recognize and compare antigens, create effector pathways that are pathogen specific and to develop a memory for antigens to counteract future infections. Cells that are involved in the adaptive immune system include; T cells, antigen presenting cells (APCs) and B cells (Warrington et al., 2014).
T cells: The cells are produced in the bone marrow, from hematopoietic stem cells (HSCs). HSCs are progenitors to all types of blood cells, meaning that they can develop into white blood cells, red blood cells, and platelets. T-cells have T-cell receptors, which utilize a unique antigen binding mechanism. They use APCs to help them recognize various antigens (Sakaguchi et al., 2008).
Mechanism of T-cells & APC antigen recognition: The activation of a T-cell begins when an APC digests an antigen and displays it on its MHC molecules. MHC, which stands for ‘major histocompatibility complex ‘ is a set of cell surface proteins that play a role within immunology and antigen recognition. After the MHC-antigen complex is formed, there is an activation of the T-cell receptor leading to secretion of cytokines by the T-cell. In addition, T-cells differentiate into either cytotoxic T cells (T-killer cells) (CD8+) or T-helper cells (CD4+) (Yamazaki et al., 2002).
CD8+ cells are involved in destroying infected cells, through the production of proteins that cause cell lysis and apoptosis. After this, most T-killer cells essentially destroy themselves and the remains are removed up by phagocytes. However, some can survive and be able to retain the information for the next time the same infection or pathogen compromises the bodies defense (Warrington et al., 2014).
T-helper cells are very important in assisting with the immune response and ensuring that it is functioning at maximum capacity. There are two main T-helper cells that can occur, Th1 and Th2. The Th1 response produced interferon-gamma which helps to activate macrophage and cytokines leading to B-cell induction. The Th2 response also releases cytokines which lead to the activation of immunoglobulin R, antibody producing B cells and mast cells (Warrington et al., 2014).
B cells: These cells are also produced in the bone marrow and are generated from HSCs. B-cells don’t need APCs to help recognize antigens, they can do this directly. The main function of these cells are to produce antibodies. When activated, they can become either plasma cells that secrete antibodies or memory B-cells (Warrington et al., 2014).
Case Study: Boy in the Bubble
David Vetter, often referred to as ‘the boy in the bubble’, was born with Severe Combined Immune Deficiency (SCID). Severe Combined Immune Deficiency is a potentially fatal primary immunodeficiency in which there is a combined absence of T-lymphocyte and B-lymphocyte function (Bosma, Custer, & Bosma, 1983). SCID is generally considered to be the most severe type of primary immunodeficiency disease and has the potential to cause extreme susceptibility to very serious infections. Affected infects are highly susceptible to recurring infections of bacteria, virus, and fungi and invariably die within 2 years of birth (Bosma G., Custer, & Bosma M., 1983).
David was born on September 21st, 1971, at the Texas Children’s Hospital (Haberman, 2015). After 20 seconds of exposure to the world, he was immediately placed in a plastic isolator bubble for protection against infections (infections that a healthy child would simply be able to shrug off). At the time of David’s birth, a bone marrow transplant from an exact matched donor was the only known cure for SCID. Unfortunately, no match was available in David’s family. David spend the rest of his 12 years of life within the sterile environment of a plastic bubble. Everything that entered his sterile environment – water, food, clothing, toys – had to be treated in a chamber filled with ethylene oxide for fours hours at 60 degrees celsius, and then aerated for a period of one to seven days before entering the bubble. Within the sterile chamber, Vetter was touched only through the use of special plastic gloves attached to the walls of his chamber. Air compressors keeping the bubble inflated were very loud and made communication with the boy difficult. However, the parents and medical team of Vetter sought to provide him as normal of a life as possible with a formal education and television inside his sterile chamber (Haberman, 2015).
In 1983, the Vetters learned of a new procedure that could allow bone marrow transfusions from donors who are not perfectly matched and agreed to try it. David received a bone marrow transport from his sister, Katherine, and although his body did not reject the transplant, he passed away four months later from lymphoma. An autopsy revealed that Katherine’s transplanted bone marrow contained traces of a dormant virus, Epstein-Barr, which at that time was not detectable by pre-transplant screening. The virus was probably responsible for David's lymphoma.
It is now known that at least 13 different genes are involved in the development of Severe Combined Immune Deficiency. Inheritance of this congenital syndrome may show X-linked or autosomal recessive control (Bosma G., Custer, & Bosma M., 1983). The most common treatment for SCID is bone marrow transplantation from tissue-matched donors. Health care professionals have also attempted gene therapy with limited success. A major limitation is the use of retroviruses to insert genetic material into the bone marrow of the child; this process has been shown to potentially cause leukaemia in later life.
Future Research & Applications
Challenges
There are many challenges that still need to be addressed within the field of Immunology. Autoimmunity is a prevalent issue that many people still suffer from. Individuals with autoimmune disorders continue to suffer from pain and inflammation due to certain immune system factors that target healthy cells instead of pathogenic cells. The immune system and how it interacts with its environment is both complex and heterogeneous, which contributes to complications that arise when treating patients with autoimmune disorders. (Olsen, 2018) Research is still needed to identify the root cause of autoimmunity and how to target pathogenic cells without harming health ones.
Next Steps
Science, in the field of Immunology, is also advancing into using combined treatments to treat diseases such as cancer. Researchers are looking at targeting multiple pathways that tumor cells use to avoid getting affected by the treatment presented. (Olsen, 2018) Along with this, scientists are investigating a combined method that allows current treatments to activate immune system factors for a stronger effect in eliminating pathogenic cells. (Olsen, 2018)
A recent review article published on The Scientist has highlighted future trends and directions in the field of Immunology. Twenty four experts were asked the question, “where do you see the most important or interesting progress occurring in immunology over the next few years?” (TheScientist, 2019). The list below highlights their responses:
List of Future Research Directions in the Field of Immunology
- Lymphokine structure and function relationships. leading through x-ray crystallography to rational drug design
- Structure of peptide and protein mediators involved in immuno-regulation and design of molecules that mimic their activity
- Mapping precisely antigenic sites and upregulation of immune response against viruses and tumor antigens
- Cellular and molecular basis for T-cell diversity
- Interactions of autoantigenic epitopes with the T-cell receptor. Solving the mystery of T-cell suppression
- Cross-reactivity between auto-antigens and microbial antigens
- Intervention in auto-immune disease by immunizing with tolerogenic autoantigens, and using monoclonal antibodies directed against critical molecules of the autoimmune response
- Signal transmission between cell receptors and gene activation
- Signal transduction of hormone receptors and identification of primary targets of mitogen-dependent protein kinases
- Recognition structures and intracellular activation signals for NK cells and other cytotoxic cells
- Signal transduction pathways of cytokines and growth factors. Biosynthesis and function of phosphatidylinositol anchors. Therapy for cancer and virus-associated diseases through biological response modifiers
- Cellular and molecular basis of immune suppression
- Therapeutic augmentation of specific immune cell subsets
- Reproducible propagation of normal T- and B-cell clones.
- Molecular basis of antigen presentation by histocompatibility antigens
(The Scientist, 2019)
References
Beck, G., & Habicht, G. S. (1996). Immunity and the Invertebrates. Retrieved from http://people.scs.carleton.ca/~soma/biosec/readings/sharkimmu-sciam-Nov1996.pdf
Bosma, G. C., Custer, R. P., & Bosma, M. J. (1983). A severe combined immunodeficiency mutation in the mouse. Nature, 301(5900), 527.
Boy in the bubble (1972). (n.d.). Retrieved from https://www.immunology.org/boy-in-the-bubble-1972
Brain, M. (2019). Different Roles- How Your Immune System Works. Retrieved from https://health.howstuffworks.com/human-body/systems/immune/immune-system11.htm
British Society for Immunology. N.d. What is immunology? Retrieved from https://www.immunology.org/public-information/what-is-immunology
Chaplin, D. D. (2010). Overview of the immune response. Journal of Allergy and Clinical Immunology, 125(2), S3-S23.
Dinarello, C. A. (2000). Proinflammatory cytokines. Chest, 118(2), 503-508.
Haberman, C. (2015, December 06). 'The Boy in the Bubble' Moved a World He Couldn't Touch. Retrieved from https://www.nytimes.com/2015/12/07/us/the-boy-in-the-bubble-moved-a-world-he-couldnt-touch.html
Hoebe, K., Janssen, E., & Beutler, B. (2004). The interface between innate and adaptive immunity. Nature immunology, 5(10), 971.
Immune Deficiency Foundation. (n.d.). Retrieved from https://primaryimmune.org/living-pi-explaining-pi-others/story-david
Immuneresponse.org. (n.d). Immune Response. Retrieved from http://www.immuneresponse.org/
Linnemeyer, P.A. (2008). The Immune System- An Overview. Retrieved from http://www.thebody.com/content/art1788.html
Medzhitov, R., & Janeway Jr, C. (2000). Innate immunity. New England Journal of Medicine, 343(5), 338-344.
National Institute of Allergy and Infectious Diseases. 2019. Overview of the Immune System. Retrieved from https://www.niaid.nih.gov/research/immune-system-overview
O'Byrne, K. J., & Dalgleish, A. G. (2001). Chronic immune activation and inflammation as the cause of malignancy. British journal of cancer, 85(4), 473.
Olsen, L. (2018). The Future is Now for Immunology Research. Retrieved from https://www.rdmag.com/article/2018/05/future-now-immunology-research
Sakaguchi, S., Yamaguchi, T., Nomura, T., & Ono, M. (2008). Regulatory T cells and immune tolerance. Cell, 133(5), 775-787.
Sela-Culang, I., Kunik, V., & Ofran, Y. (2013). The structural basis of antibody-antigen recognition. Frontiers in immunology, 4, 302.
Stossel, T. P. (1974). Phagocytosis. New England Journal of Medicine, 290(13), 717-723.
The Scientist. (2019). 24 Experts Forecast Future Trends In Immunology. Retrieved from https://www.the-scientist.com/research/24-experts-forecast-future-trends-in-immunology-62715
Warrington, R., Watson, W., Kim, H. L., & Antonetti, F. R. (2011). An introduction to immunology and immunopathology. Allergy, Asthma & Clinical Immunology, 7(1), S1.
Yamazaki, T., Akiba, H., Iwai, H., Matsuda, H., Aoki, M., Tanno, Y., … & Azuma, M. (2002). Expression of programmed death 1 ligands by murine T cells and APC. The Journal of Immunology, 169(10), 5538-5545.