Table of Contents
Hyperbilirubinemia: Jaundice
LS4M03 ppt link: https://docs.google.com/presentation/d/17I4UVA_GAagsoywBAQvyIQ6UZCuQBjTS8bzAhAupoAA/edit?usp=sharing
Introduction
 Jaundice (resulting from neonatal hyperbilirubinemia) is one of the most common conditions that occurs shortly after birth. It is primarily identified by the yellowing of the baby’s skin, often extending down through the abdomen, and to the arms and legs. Usually, this is accompanied by the yellowing of the sclera. About 60% of term and 80% of preterm babies develop jaundice in the first week of life, and about 10% of breastfed babies are still jaundiced at 1 month of age (Rennie, Roy, & Murphy, 2010). The development of jaundice shortly after birth is known as “physiological jaundice” (Maisels, 2006). Prior to ligation of the umbilical cord, the infant’s bilirubin load is cleared through the placenta. However, following ligation, the neonate must dispose of the bilirubin through individual mechanisms that could often be defective or under developed. These mechanisms include a decreased hepatic uptake of bilirubin, decreased bilirubin conjugation, and defective bilirubin excretion (Maisels, 2006). These defective mechanisms in the neonate result in a transient elevation of unconjugated plasma bilirubin. Unconjugated bilirubin is the conversion of heme to unconjugated bilirubin, which is tightly bound to albumin. Normally, unconjugated plasma bilirubin levels in physiologic jaundice does not exceed 100 µmol/L (Marshall, Bangert & Lapsley, 2012).
Jaundice (resulting from neonatal hyperbilirubinemia) is one of the most common conditions that occurs shortly after birth. It is primarily identified by the yellowing of the baby’s skin, often extending down through the abdomen, and to the arms and legs. Usually, this is accompanied by the yellowing of the sclera. About 60% of term and 80% of preterm babies develop jaundice in the first week of life, and about 10% of breastfed babies are still jaundiced at 1 month of age (Rennie, Roy, & Murphy, 2010). The development of jaundice shortly after birth is known as “physiological jaundice” (Maisels, 2006). Prior to ligation of the umbilical cord, the infant’s bilirubin load is cleared through the placenta. However, following ligation, the neonate must dispose of the bilirubin through individual mechanisms that could often be defective or under developed. These mechanisms include a decreased hepatic uptake of bilirubin, decreased bilirubin conjugation, and defective bilirubin excretion (Maisels, 2006). These defective mechanisms in the neonate result in a transient elevation of unconjugated plasma bilirubin. Unconjugated bilirubin is the conversion of heme to unconjugated bilirubin, which is tightly bound to albumin. Normally, unconjugated plasma bilirubin levels in physiologic jaundice does not exceed 100 µmol/L (Marshall, Bangert & Lapsley, 2012).
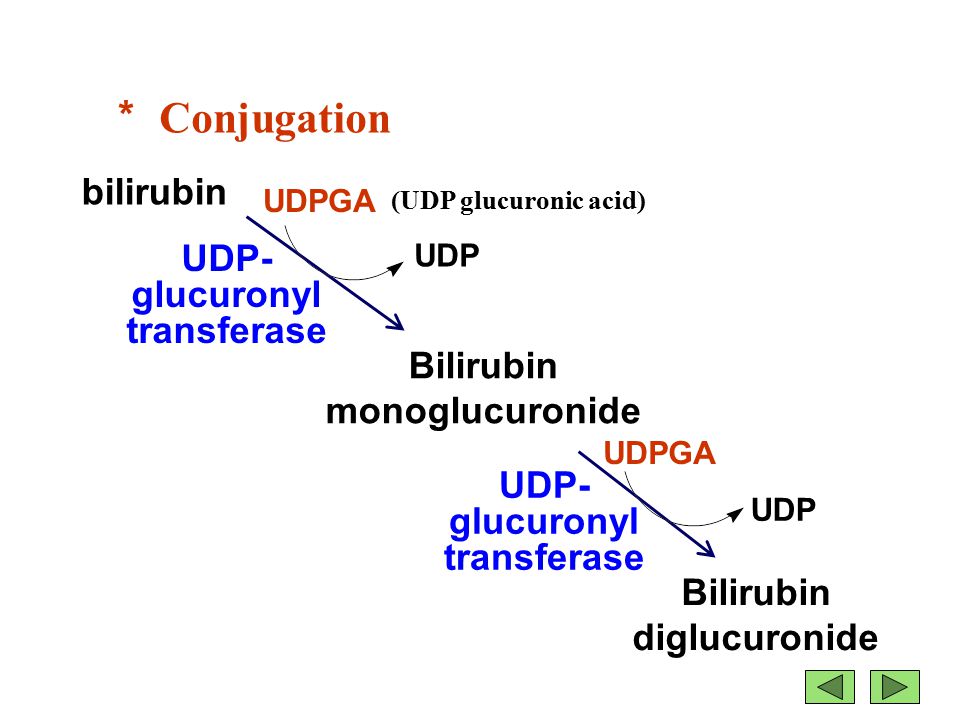
Figure 1: The conjugation of bilirubin by uridine diphosphate glucuronosyltransferase (UGT) to bilirubin diglucuronide (conjugated form).
Focus: Breast Milk Jaundice
Breastfed babies have a greater risk for developing jaundice due to the “breast milk jaundice” phenomenon. Literature speculates that a compound in breast milk is responsible for inhibiting the normal conjugation of bilirubin, therefore resulting in elevated unconjugated plasma bilirubin. This compound, 3α,20β- Pregnanediol, inhibits glucuronosyltransferase which is an enzyme that is responsible for the normal conjugation of bilirubin (Hargreaves & Piper, 1971).
Focus: Kernicterus
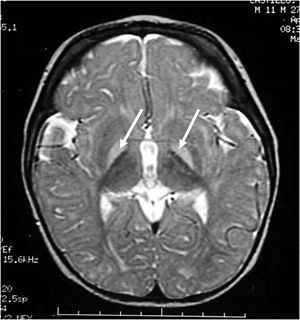 This neurologic condition, also known as bilirubin encephalopathy, is characterized by the deposition of unconjugated bilirubin within the brain (>350 µmol/L). In the plasma, bilirubin is usually bound to albumin, rendering it nontoxic (Kriss, Kollias, & Ball, 1995). However, when the concentration of bilirubin exceeds normal albumin binding capacity, free unconjugated bilirubin enters the brain and proposes toxic effects. This is characterized by hypotonia, rigidity, and a high-pitched cry. Early into the disease, symptoms may be subtle. However, once neurologic symptoms appear, the prognosis is poor. Currently, blood transfusions along with increased feedings are implemented in order to reduce the bilirubin levels (Kriss, Kollias, and Ball, 1995).
This neurologic condition, also known as bilirubin encephalopathy, is characterized by the deposition of unconjugated bilirubin within the brain (>350 µmol/L). In the plasma, bilirubin is usually bound to albumin, rendering it nontoxic (Kriss, Kollias, & Ball, 1995). However, when the concentration of bilirubin exceeds normal albumin binding capacity, free unconjugated bilirubin enters the brain and proposes toxic effects. This is characterized by hypotonia, rigidity, and a high-pitched cry. Early into the disease, symptoms may be subtle. However, once neurologic symptoms appear, the prognosis is poor. Currently, blood transfusions along with increased feedings are implemented in order to reduce the bilirubin levels (Kriss, Kollias, and Ball, 1995).
Figure 2: Bilirubin-induced lesions in the basal ganglia.
Epidemiology
Jaundice is most prevalent in premature infants, with 80% of them developing the disease, typically 2-4 days after birth. Data in the United States showed 50% of full-term infants developed the disease after 2-4 days after birth and in both cases; the disease disappeared in 1 to 2 weeks. It is also seen that newborns have a serum bilirubin level that is higher than adults (Woodgate & Jardine, 2011). Unfortunately, there is currently no reliable data on incidents of jaundice of adult populations due to the lack of appropriate sample size (Chu, 2014).
The standard of living appears to have an effect on the prevalence of the disease, as developing countries are more susceptible to severe cases of jaundice. A study from Turkey reported significant jaundice in 10.5% of full-term infants and in 25.3% of near-term infants (Sarici et al., 2004). In contrast, significant jaundice occurs in 8% of newborns in the United States (Woodgate, P & Jardine, L. A., 2011). In the 1994 American Academy of Pediatrics (AAP) guidelines, it was noted that 4.3% of 47,801 infants had total serum bilirubin levels in a range where phototherapy was recommended (Atkinson et al., 2003).
Interestingly, another risk factor that influences the prevalence of developing jaundice is racial background. Reports indicated that East Asians have higher bilirubin levels at birth than Caucasians. Moreover, 31% of East Asian infants have an approximately 3-fold increased risk of acquiring the disease compared to African/African Americans where incidence is much lower (Setia et al., 2002).
Major Functions of the Liver
Due to the intricate relationship between the liver and biliary system, jaundice is a frequent feature of liver disease (Marshall, Bangert & Lapsley, 2012). The liver plays a pivotal role in metabolism and in the detoxification and elimination of toxic substances (Marshall, Bangert & Lapsley, 2012). Damage to this organ may not directly affect its activity as the liver has several functional reserves, but it can propose serious life-threatening diseases (Marshall, Bangert & Lapsley, 2012). Some of the major functions of the liver are outlined below (Marshall, Bangert & Lapsley, 2012).
- Carbohydrate Metabolism
- Gluconeogenesis
- Glycogen synthesis and breakdown
- Fat Metabolism
- Fatty acid synthesis
- Cholesterol synthesis and excretion
- Lipoprotein synthesis
- Ketogensis
- Bile acid synthesis
- 25-hydroxylation of vitamin D
- Protein Metabolism
- Synthesis of plasma proteins (i.e., some coagulation factors)
- Urea synthesis from ammonia
- Hormone Metabolism
- Metabolism and excretion of steroid hormones
- Metabolism of polypeptide hormones
- Storage
- Glycogen
- Vitamin A
- Vitamin B12
- Iron
- Drugs and Foreign Compounds
- Metabolism and excretion
- Metabolism and Excretion of Bilirubin
Biochemical Assessment of Liver Function
The diagnosis of liver disorders involves a biochemical assessment of liver function. Clinically speaking, they are referred to as Liver Function Tests (LFTs). LFTs are a series of blood tests that can be examined to indicate the state of a patient's liver. The primary analytes of LFTs are various proteins in the blood plasma. Outlined below are a few different types of liver function tests.
Alanine Transaminase (ALT) Test
ALT is an enzyme that catalyzes the breakdown of proteins and is found mainly in the liver. High plasma concentrations of ALT can be indicative of severe liver damage or liver disease. The reference range for normal levels are between 20-60 IU/L. Pathological reference ranges can be up to 1000 IU/L (Marshall, Bangert, Lapsley, 2012).
Alkaline Phosphatase (ALP) Test
ALP is an catabolic enzyme found in the lining of bile ducts in the liver, and also in some bone tissue. The normal reference range is between 30-120 IU/L. It is normal to find slightly elevated levels in growing children, however, significantly elevated levels can be indicative of a bile duct obstruction (tumour), liver damage or disease, or bone disease. The ALP test is often done in combination with the ALT test to confirm liver disease in children and adults (Marshall, Bangert & Lapsley, 2012).
Albumin and Total Protein Test
Albumin and globulin are the most commonly synthesized proteins of the liver. Mainly functioning as carrier proteins in the bloodstream, low levels are highly indicative of liver cirrhosis. Normal albumin levels range from anywhere between 3.5-5.5 g/dL. Liver disease in children can greatly reduce numbers to under 2 g/dL indicative of early-onset hepatocellular carcinoma (Marshall, Bangert & Lapsley, 2012).
Bilirubin Test
The bilirubin test is the most commonly performed test for confirming the diagnosis of jaundice. The protein of interest in plasma is the metabolite bilirubin in its unconjugated form (Marshall, Bangert & Lapsley, 2012). Bilirubin is a metabolite derived from erythrocyte catalysis. Healthy, optimally functioning livers maintain a normal range of 0.1-1 mg/dL for children under 18 years and 1.2 mg/dL for adults. A plasma bilirubin concentration of 3 mg/dL is the approximate level in which yellowing of the skin or eyes is detectable, indicating jaundice (Marshall, Bangert & Lapsley, 2012).
Gamma-Glutamyl Transpeptidase (GGT) Test
Reference range of GGT is between 0-30 IU/L in healthy individuals. GGT is present in the membranes of cells within the lining of the bile duct and abnormally high levels of GGT can be indicative of liver disease, bile duct damage, or a tumour (Marshall, Bangert & Lapsley, 2012).
Prothrombin time (PT) test
This test measures how long it takes your blood to clot. The average time for healthy individuals ranges from 25-30 seconds. A longer clotting time could be a sign of liver damage. However, medications such as blood thinners can prolong clotting time (Marshall, Bangert, Lapsley & 2012)
Urine Analysis as an Assessment of Liver Function
Urine analysis can also be used as a method of screening for liver disorders. The main biomarkers of interest for assessing liver function include bilirubin and urobilinogen. If both of these biomarkers mentioned above are elevated greater than baseline (0 for infants), a physician will certainly order more laboratory tests to confirm the diagnosis of specific liver dysfunction.
Presence of Bilirubin
In normal healthy individuals, bilirubin is not present in the urine even in trace amounts since it is a waste product produced by the liver from erythrocyte catalysis. Accelerated catalysis can lead to an accumulation of bilirubin in the urine (conjugated form). This can be an early indicator of liver dysfunction even prior to the development of clinical symptoms of jaundice (Marshall, Bangert & Lapsley, 2012). Hyperbilirubinuria, the abnormal presence of excess bilirubin (conjugated) in the urine is always considered pathological (Marshall, Bangert & Lapsley, 2012).
Presence of Urobilinogen
Urobilinogen is normally present in the urine in low concentrations. It is formed in the intestine from bilirubin. Elevated levels can indicate liver diseases such as viral hepatitis, cirrhosis, liver damage due to drugs or toxins. When urobilinogen in the urine are low or absent in a person with hyperbilirubinuria and or signs of liver dysfunction, it can indicate the presence of a hepatic or biliary obstruction (Marshall, Bangert & Lapsley, 2012).
Bilirubin Metabolism
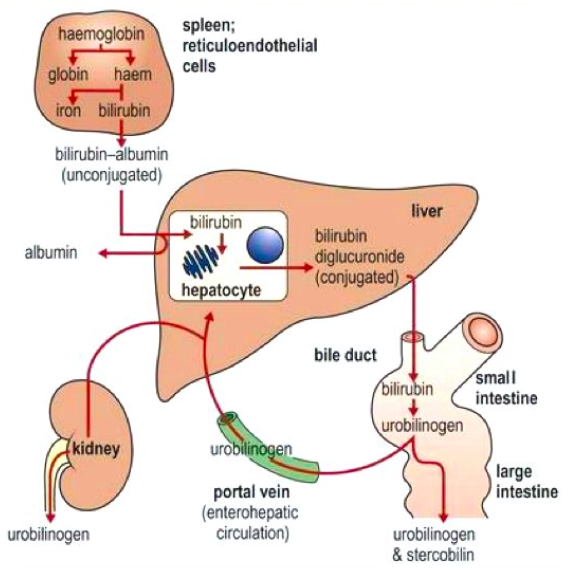
Bilirubin is mainly formed from the catabolic breakdown of the heme moiety present in hemoglobin molecules (Marshall, Bangert & Lapsley, 2012). Other sources of bilirubin include myoglobin and cytochromes (Marshall, Bangert & Lapsley, 2012). In the plasma, unconjugated bilirubin is transported in the bloodstream tightly bound to albumin to prevent its excretion in the urine (Wang, Chowdhury J & Chowdhury N, 2006). Due to the internal hydrogen bonding, unconjugated bilirubin is water-insoluble and requires enzyme-mediated glucuronidation, a form of conjugation, in the liver in order to be excreted (Wang, Chowdhury J & Chowdhury N, 2006). Unconjugated bilirubin is taken up into hepatocytes and conjugated to glucuronides in the smooth endoplasmic reticulum to form mono- and diglucuronides (Marshall, Bangert & Lapsley, 2012; Wang, Chowdhury J & Chowdhury N, 2006). This process is catalyzed by the enzyme bilirubin uridine diphosphate glucuronosyltransferase (UGT) which results in a conjugated, water-soluble form of bilirubin that is excreted via the bile ducts and further converted to urobilinogen (Marshall, Bangert & Lapsley, 2012; Wang, Chowdhury J & Chowdhury N, 2006). The specific isoform of the UGT enzyme, known as UGT1A1, is the only enzyme capable of performing the chemical reaction called glucuronidation (National Library of Medicine, 2017). This enzyme converts the toxic form of bilirubin (unconjugated bilirubin) to its nontoxic form (conjugated bilirubin), making it able to be dissolved and removed from the body (National Library of Medicine, 2017). The bilirubin-UGT enzyme is primarily found in cells of the liver, where bilirubin glucuronidation takes place, more specifically in the smooth endoplasmic reticulum (National Library of Medicine, 2017).
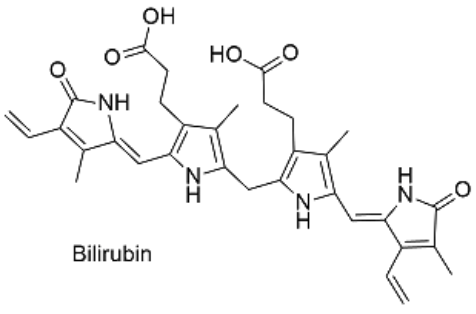
Figure 3: Excretion of bilirubin (structure on right) by the liver (Marshall, Bangert & Lapsley, 2012; google images)
Pathophysiology of Bilirubin Metabolism
A healthy liver can metabolize and excrete ten times the normal amount of bilirubin (300mg) that is produced daily (Marshall, Bangert & Lapsley, 2012). Therefore, measurements of plasma bilirubin concentrations are considered insensitive tests of liver function (Marshall, Bangert & Lapsley, 2012). The bilirubin that is normally present in the plasma is about 95% unconjugated (Marshall, Bangert & Lapsley, 2012). Since it is protein bound and water-insoluble, unconjugated bilirubin is not filtered by the renal glomeruli and is usually not detected in the urine (Marshall, Bangert & Lapsley, 2012). Therefore, the abnormal presence of bilirubin in the urine reflects a high concentration of conjugated bilirubin in the plasma and is always pathological (Marshall, Bangert & Lapsley, 2012; Wang, Chowdhury J & Chowdhury N, 2006). Although jaundice is a frequent feature of liver disease, clinical manifestations may not be as obvious until plasma bilirubin concentrations are more than twofold the upper limit of normal, which is 50 μmol/L (Marshall, Bangert & Lapsley, 2012).
Several factors may lead to problems in bilirubin metabolism. Elevated levels of unconjugated bilirubin can pass the blood brain barrier and pose neurotoxic effects in the brain as well as cause a yellow-orange discolouration (jaundice) of an individual's skin, eyes, and other tissues (Yaworski, Meer & Wong, 2015). Abnormal levels of bilirubin in the plasma may be of pre-hepatic, hepatic, post-hepatic origin or a genetic defect. The different effects of perturbed bilirubin metabolism are outlined below.
Inherited Abnormalities of Bilirubin Metabolism
The conjugation (attachment) of glucuronides to bilirubin is crucial for biliary excretion by the liver (Wang, Chowdhury J & Chowdhury N, 2006). Bilirubin glucuronidation is catalyzed by a specific isoform of uridine diphosphsphate glucuronosyltransferase, termed UGT1A1 (Wang, Chowdhury J & Chowdhury N, 2006). There are four conditions in which jaundice (hyperbilirubinemia) is caused by an inherited abnormality of bilirubin metabolism. These include: Gilbert's, Crigler-Najjar, Dubin-Johnson, and Rotor syndromes (Marshall, Bangert & Lapsley, 2012). The most common syndrome among the fours listed is Gilbert's syndrome, which affects about 5-7% of the population (Marshall, Bangert & Lapsley, 2012).
Focus: Gilbert's Syndrome
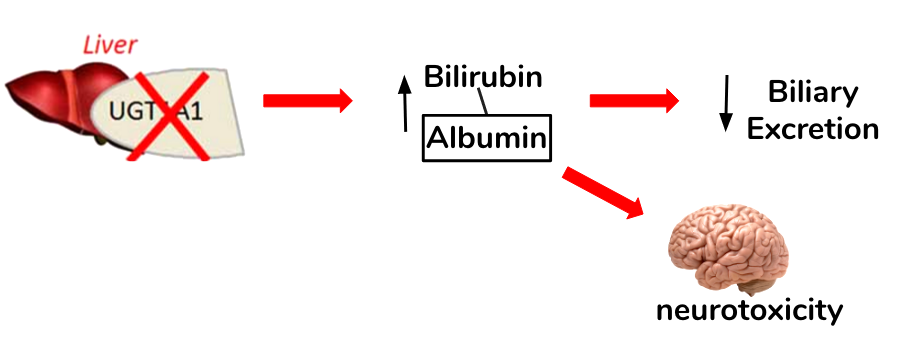
The autosomal recessive syndrome is inherited by a mutation in the promoter for the UDP-glucuronosyl transferase gene (UGT1A1), which leads to a decrease in gene expression and thus enzyme activity (Marshall, Bangert & Lapsley, 2012). Normally, this enzyme functions to perform an important chemical reaction called glucuronidation in which a compound called glucuronic acid is conjugated to bilirubin in order for biliary excretion by the liver (Burchell, Brierley, Monaghan, & Clarke, 1997; Guillemette, 2003). A defect in the enzyme, then, decreases conjugation of bilirubin and uptake in some cases leading to mild but fluctuating unconjugated hyperbilirubinemia (Marshall, Bangert & Lapsley, 2012). Gilbert's syndrome is characterized by the absence of liver disease and episodes of mild intermittent jaundice (Guillemette, 2003).
Figure 4: The loss of function in the UGT1A1 and its implications in bilirubin conjugation and clearance. Elevated levels of unconjugated bilirubin possess neurotoxic properties in the brain.
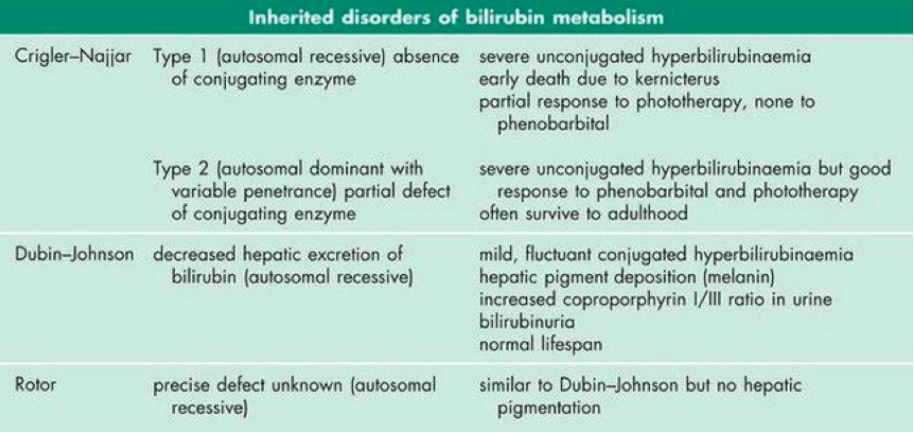
The remaining inherited disorders of bilirubin metabolism are quite rare. Their defects and clinical features are outlined below in figure 5.
Figure 5: Rare inherited disorders of bilirubin metabolism, their defects and clinical features (Marshall, Bangert & Lapsley, 2012)
Causes of Hyperbilirubinemia (Jaundice)
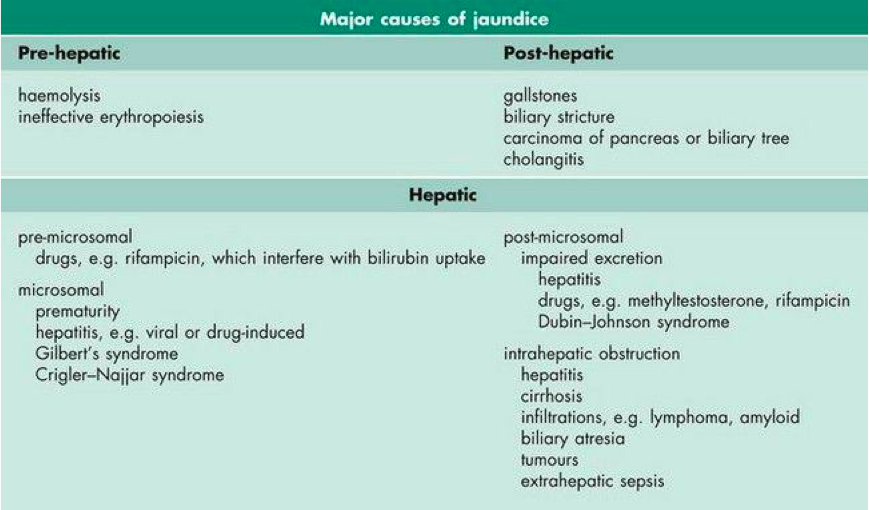
The yellow-orange discolouration of the skin in patients with jaundice is generally caused by a high plasma concentration of bilirubin (Marshall, Bangert & Lapsley, 2012). Jaundice is a characteristic sign and symptom of a liver disease (Marshall, Bangert & Lapsley, 2012). Hyperbilirubinemia can be caused either by excess conjugated or unconjugated bilirubin (Marshall, Bangert & Lapsley, 2012). Some of the other causes of hyperbilirubinemia include increased production of bilirubin and impaired conjugation or excretion of bilirubin (Marshall, Bangert & Lapsley, 2012; (Yaworski, Meer & Wong, 2015). Since bilirubin is pigmented and has colour, it causes a yellow-orange colour of an individual's skin, eyes and other tissues (Standford Children's Health, 2017). Jaundice is usually a manifestation of bilirubin accumulation from hemolysis due to the breakdown of red blood cells (in neonates, due to a hemolytic disease such as Rh disease) or liver dysfunction (Standford Children's Health, 2017). Furthermore, figure 6 below outlines some of the major causes of jaundice that occur prior to the liver (pre-hepatic) resulting from hemolysis which is red blood cell catalysis, after the liver (post-hepatic) meaning the pathology is occurring after the conjugation of bilirubin possibly because of biliary obstruction or damage, and hepatic meaning the pathology is located within the liver for instance genetic defects or diseases of the liver cells (Marshall, Bangert & Lapsley, 2012).
Figure 6: Classification and some of the major causes of jaundice (hyperbilirubinemia) (Marshall, Bangert & Lapsley, 2012)
Focus: Neonatal Hyperbilirubinemia
Jaundice is common in neonates and several factors specific to their physiology contribute to hyperbilirubinemia (Marshall, Bangert & Lapsley, 2012; Yaworski, Meer & Wong, 2015). Infants are not easily able to get rid of the bilirubin causing it to accumulate in the blood, other tissues and fluids of the neonate's body (Stanford Children’s Health, 2017). Such jaundice is usually due to the accumulation of unconjugated bilirubin (Marshall, Bangert & Lapsley, 2012). Increased production of bilirubin, deficiency of hepatic uptake, impaired conjugation of bilirubin, and increased enterohepatic circulation of bilirubin account for some of the other cases of jaundice in newborn infants (Dennery, Seidman, & Stevenson, 2001). Two main factors that lead to neonatal hyperbilirubinemia are outlined below (Yaworski, Meer & Wong, 2015).
- Increased Production of Bilirubin
- Turnover rates for fetal red blood cells are much higher and newborn infants, per kilogram, produce twice the daily adult amount of bilirubin. Abnormally high levels of bilirubin in the bloodstream can be harmful to the brain.
- Decreased Clearance of Bilirubin
- Newborns have relatively low UDP glucuronosyltransferase activity, thus their ability to conjugate bilirubin is limited. Also, their intestinal motility is very slow in the first few days as feeding becomes established, and this increases small amounts of bilirubin re-uptake by the enterohepatic circulation.
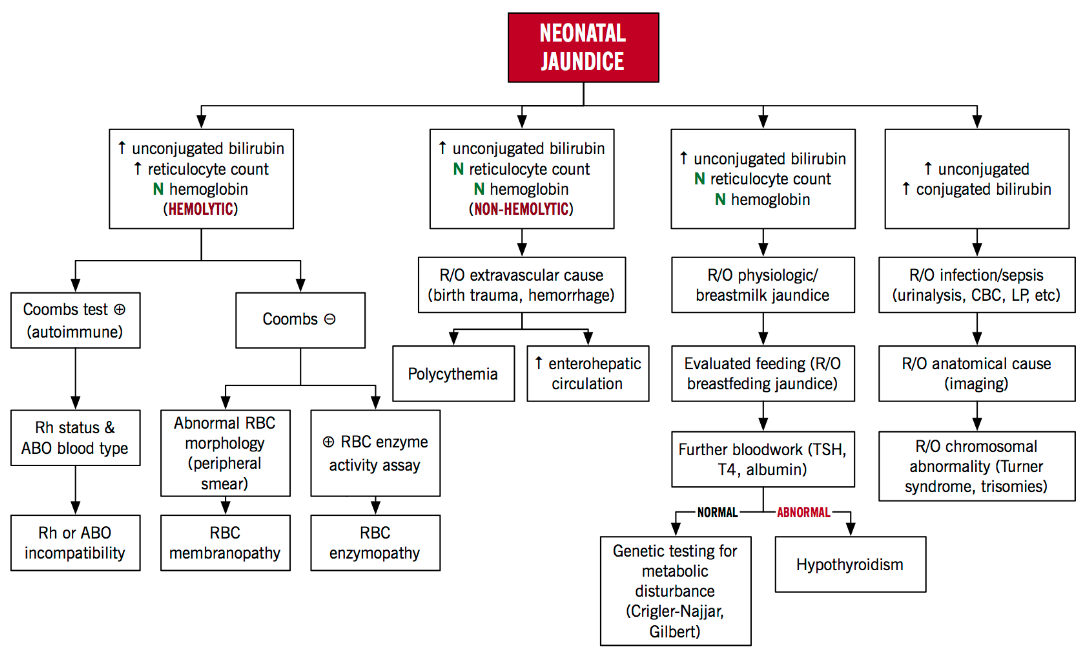
Figure 7 below highlights the two main factors that lead to neonatal jaundice and other causes of hyperbilirubinemia that also lead to neonatal jaundice. These causes can be categorized in to hemolytic and non-hemolytic mechanisms. Hemolysis results in greater levels of bilirubin than hepatic conjugation is able to keep pace with. In this case, the total unconjugated bilirubin load is increased. Rh factor incompatibility can cause lysis of fetal red blood cells increasing bilirubin load in the blood (Yaworski, Meer & Wong, 2015). When an Rh-negative woman carries an Rh-positive fetus, she may develop antibodies to the Rh antigen on fetal red blood cells (Yaworski, Meer & Wong, 2015). These maternal anti-Rh antibodies cross the placenta and induce lysis of fetal red blood cells. Non-hemolytic causes include enterohepatic circulation in which the bilirubin is not excreted and circulates from the liver to the intestines, physiologic hyperbilirubinemia in which the neonatal liver cannot efficiently conjugate bilirubin and breast milk jaundice in which a compound in breast milk impairs normal conjugation of bilirubin (Yaworski, Meer & Wong, 2015).
Figure 7: Neonatal Hyperbilirubinemia Algorithm: An Approach to Neonatal Jaundice (Yaworski, Meer & Wong, 2015)
Diagnosis
There are three categories of jaundice, each of which have an underlying disease that is causing the disruption leading to different diagnoses . These are outlined below (National Health Services, 2015).
- Pre-hepatic jaundice: the disruption occurs prior to the transportation of blood to the liver. There is excessive red blood cell breakdown
- Intra-hepatic jaundice: the disruption occurs in the liver as it loses its ability to conjugate bilirubin
- Post-hepatic jaundice: referred to as “obstruction of biliary drainage”. This is where there is a disruption in the drainage of the bile that has bilirubin inside
Urine Test
A urine Test may be performed to measure urobilinogen levels in an individual’s urine sample. Typically, urobilinogen is present in levels of <17 μmol/l in healthy individuals (National Institute of Health, 2017).
Individuals that show low levels of urobilinogen in the urine may be an indication of post-hepatic jaundice and could be the result of:
- Congestion in the pathway that carries bile from your liver
- Clogged blood flow in the liver
- A disruption with liver function
High level of urobilinogen may be an indication of pre-hepatic jaundice or intra-hepatic jaundice and be could be the result of:
- Hepatitis
- Cirrhosis
- Liver damage due to drugs
- Hemolytic anemia
Liver Function Test
LFTs are a type of blood test that measure enzyme and protein levels related to the liver. There should be in an increase in enzyme levels when a liver is damaged as enzymes are released into circulation. However, there should also be a decrease in levels of proteins that the liver produces to keep the body healthy (National Health Services, 2015).
The different types of LFTs can be found under the “Biochemical Assessment of Liver Function” section.
Imaging Testing
Individuals who are suspected to have intra-hepatic jaundice or post-hepatic jaundice will be put through image testing to check for abnormalities. These include:
Ultrasonography: This procedure is the use of high frequency sounds wave to produce images of internal organs and tissue. It is widely considered the most useful first line of investigation in individuals with jaundice. It is non-invasive and inexpensive. It is reliable for identifying gallbladder stones and defects in the liver such as cysts, tumours and fluids aggregation (Beckingham & Ryder, 2001).
Computed tomography (CT): CT scans are testing that involves the combination of x-ray images taken at different angles to create a cross-sectional image. Through this test, it can provide information related to liver texture, bile duct dilation, gallbladder and pancreatic diseases (Beckingham & Ryder, 2001).
Endoscopic retrograde cholangiopancreatography (ERCP): The process involves using a small, flexible camera to inject a dye into the bile ducts. This allows for better visualization on X-ray as the dye would be prominent. It is useful in helping to confirm a diagnosis of pre-hepatic jaundice (National Institute of Health, 2017).
Liver Biopsy
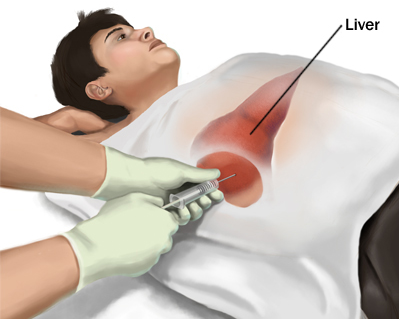 Traditionally, the process involves inserting a small needle in the liver to collect tissue sample. A biopsy is recommended to examine the status of liver tissue if physicians feel the liver may have taken damage from a condition such as liver cancer or cirrhosis (National Institute of Health, 2017). There are incidents of bile leaks and haemorrhages, however the overall mortality rate from this procedure is low at 0.1% (Beckingham & Ryder, 2001). Furthermore, liver biopsies can be done by ultrasound as a guide to diagnosing liver masses. However, there are many complications that may arise such as bleeding, anaphylactic shock or tumour seeding (Beckingham & Ryder, 2001).
Traditionally, the process involves inserting a small needle in the liver to collect tissue sample. A biopsy is recommended to examine the status of liver tissue if physicians feel the liver may have taken damage from a condition such as liver cancer or cirrhosis (National Institute of Health, 2017). There are incidents of bile leaks and haemorrhages, however the overall mortality rate from this procedure is low at 0.1% (Beckingham & Ryder, 2001). Furthermore, liver biopsies can be done by ultrasound as a guide to diagnosing liver masses. However, there are many complications that may arise such as bleeding, anaphylactic shock or tumour seeding (Beckingham & Ryder, 2001).
Figure 8: Liver biopsy procedure to check the status of liver function in an individual
Complications
Elevated levels of bilirubin that cause severe jaundice can result in serious complications if left untreated. While jaundice is a very treatable and tolerable disorder, in very rare cases individuals who do not receive proper care due to limited access to healthcare resources (a consequence in many developing nations), long-term effects on the newborn's body can be quite detrimental.
Acute Bilirubin Encephalopathy
Bilirubin is highly toxic to cells of the brain. Given an infant's immature blood-brain barrier, if a baby has severe jaundice there is a risk of bilirubin passing into the brain, a condition called acute bilirubin encephalopathy. Prompt treatment may prevent significant lasting damage. These are outlined below.
- Listlessness or difficulty waking
- High-pitched crying
- Poor sucking or feeding
- Backward arching of the neck and body
- Fever
- Vomiting
Kernicterus
Kernicterus is the syndrome that occurs if acute bilirubin encephalopathy causes permanent damage to the brain. Kernicterus may result in:
- Involuntary and uncontrolled movements (athetoid cerebral palsy)
- Permanent upward gaze
- Hearing loss
- Improper development of tooth enamel
Treatment and Prevention
Phototherapy
Currently, the most popular and effective intervention for neonatal hyperbilirubinemia is phototherapy. Phototherapy is a treatment which exposes a patient to light energy via lamps. The reason this form of therapy is used in patient's with jaundice is because it has been shown that when unconjugated bilirubin is exposed to light energy, its structure changes to a lighter and less lipophilic stereoisomer which can be excreted in bile or urine without conjugation (Lightner & McDonagh, 1984). This process of bilirubin’s structural change through light exposure is called photoisomerization (Lightner & McDonagh, 1984). The bilirubin circulating in the dermal and subcutaneous areas, or the superficial capillaries and interstitial spaces, encounters the light and photochemical reactions are performed. Moreover, structural and configurational isomerization and photo-oxidation transforms the unconjugated bilirubin into a water-soluble and non-toxic isomer making them excretable (McDonagh, 2001). This form of treatment can be very helpful for individuals with reduced bilirubin-UGT enzyme activity, in which their ability to conjugated is limited 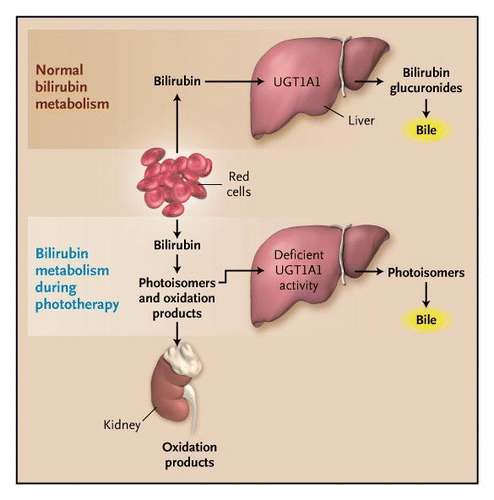
There are four factors that influence the efficacy of phototherapy: the wavelength of light, the irradiance (intensity) of light, the distance between the light source and skin, and the surface area of the body exposed to light. The most effective wavelength of light in degrading bilirubin is in the range of 400-520 nm, with the optimal wavelength being around 460 nm – which is blue light (Vreman, Wong & Stevenson, 2004). Blue light strikes the best balance between penetration of the skin and absorption by the unconjugated bilirubin (Maisels, 2005). Intuitively, there is a strong correlation between the intensity of light used, the surface area of skin exposed to light, short distances between the light source and body, and the rate at which bilirubin levels decline (Tan, 1982).
Phototherapy is mainly used as a treatment for neonatal jaundice to prevent kernicterus and potentially irreversible brain damage. When babies are born, their hepatocytes may not be entirely functional yet which prevents sufficient excretion of bilirubin. Since the blood-brain barriers of neonates are still weak, an excess of free unconjugated bilirubin circulating in the blood may pass through and cause neurologic damage to the brain (Kriss, Kollias & Ball, 1995). In contrast to babies, phototherapy is not performed on adults with jaundice since their hyperbilirubinemia is usually a result of hemolysis, chronic hepatitis, liver damage through alcohol, and other diseases of the liver. Furthermore, the condition causing the clinical symptoms of jaundice is treated in adults instead of the actual jaundice itself.
Figure 9: The effect of phototherapy in individuals with deficient UGT activity and decreased ability to conjugate and excrete bilirubin.
Exchange Transfusions
Blood exchange transfusion consists of slowly replacing the recipient’s blood with donor blood, which helps to lower the bilirubin levels. This once was the main treatment for neonatal jaundice until phototherapy was discovered. Exchange transfusion is rare in more developed countries, and it is only used in emergencies of severe hyperbilirubinemia when phototherapy is not proving to be adequately fast (Murki & Kumar, 2011). Prenatal and postnatal care has also significantly improved over time, which has been shown to be a factor in the decline of the frequency of exchange transfusions (Steiner, Bizzarro, Ehrenkranz, et. al, 2007). On the other hand, exchange transfusions are still quite common in developing countries that lack sufficient resources for phototherapy (Murki & Kumar, 2011).
Prevention
Studies with rats show fasting increases enterohepatic circulation of bilirubin because of the reduced intestinal motility and increased reuptake of bile pigments into blood circulation (Kotal, Vitek, & Fevery, 1996). Another study, this time with neonates with hyperbilirubinemia undergoing phototherapy, shows that being fed with breast milk along with supplemental formula improved the efficacy of phototherapy more than neonates fed with only formula or only breast milk (Tan, 1998). The above findings imply more frequent feeding of neonates, especially with breast milk and formula supplements, can act as a somewhat preventative measure from severe hyperbilirubinemia and can quicken the treatment process.
Implications & Future Research
Protective Effects of Mild Hyperbilirubinemia
Interestingly, bilirubin is a strong antioxidant and mild hyperbilirubinemia may have a protective effect against ischemic cardiovascular disease and cancer (Wang, Chowdhury J & Chowdhury N, 2006). In fact, a recent study looking at the history of colorectal cancer done on a large population reported the odds ratios to be reduced to 0.295 in men and 0.186 in women per 1 mg/dl increment in serum bilirubin levels (Wang, Chowdhury J & Chowdhury N, 2006). Furthermore, an inverse relationship between serum bilirubin levels and cancer mortality has also been reported (Wang, Chowdhury J & Chowdhury N, 2006). However, these findings do not suggest a direct cause-and-effect relationship and more research needs to be done in this area.
Focus: Stanate (Stannsoporfin)
Stanate (Stannsoporfin) is in late-stage development at WellSpring pharmaceuticals and is being used for the treatment of neonatal jaundice and prevention of kernicterus (“Compassionate Use of Stanate ™ “, 2013). Stanate’s mechanism of action specifically inhibits the enzyme that blocks the conversion of heme into bilirubin. Since Kernicterus is attributed to high levels of bilirubin, this drug is aimed to stop this excess production. Tin mesoporphyrin IX dichloride is the chemical compound that is being investigated for use in infant jaundice. This drug, given by injections, specifically targets heme oxygenase 1 and 2, which are involved in the cleavage of heme in order to be converted to bilirubin. It is mainly executed when the neonate has very high bilirubin levels and is failing phototherapy. Also, it is used when a family refuses exchange blood transfusion for religious purposes. The results of the clinical trial demonstrated that the use of this drug significantly lowered blood bilirubin levels, and resulted in fewer and shorter uses of phototherapy, compared with placebo (“Compassionate Use of Stanate ™ “, 2013).
Focus: Glycerin suppositories
Glycerin suppositories are a form of laxatives, normally used to relieve constipation. A study using glycerine suppositories was conducted to determine if it would reduce jaundice in premature infants with respect to decreasing exposure to phototherapy (“Glycerin Suppositories to Reduce Jaundice in Premature Infants”, 2012). This randomized, double-blind study incorporated 75 premature babies with physiological hyperbilirubinemia between the ages of 30-35 weeks. This was developed based on previous research showing that delayed meconium (earliest stool of infant) evacuation is a contributing factor to persistent neonatal jaundice. Several studies have been performed, but the results are inconclusive. One study found that a subgroup of male infants with blood group type A displayed a lower mean total serum bilirubin level after induction of earlier meconium with glycerine suppositories. However, in a different study, randomized groups were given glycerine at 12 hours of life, and bilirubin levels were followed for the first 7 days of life. This did not have an effect on serum bilirubin levels in the first 7 days. In the clinical trial through the University of Rochester, total serum bilirubin levels dropped by 0.7 mg/dL when glycerine suppository was employed (“Glycerin Suppositories to Reduce Jaundice in Premature Infants”, 2012). This trial has now been completed, and glycerin being mainly used for constipation, as no concrete evidence fully supports this method in regards to neonatal jaundice.
References
Atkinson, L. R., Escobar, G. J., Takayama, J. I., & Newman, T. B. (2003). Phototherapy use in jaundiced newborns in a large managed care organization: do clinicians adhere to the guideline?. Pediatrics, 111(5), e555-e561. Beckingham, I. J., & Ryder, S. D. (2001). Investigation of liver and biliary disease. BMJ : British Medical Journal, 322(7277), 33–36.
Burchell, B., Brierley, C. H., Monaghan, G., & Clarke, D. J. (1997). The Structure and Function of the UDP-Glucuronosyltransferase Gene Family. In D. S. Goldstein, G. Eisenhofer, & R. McCarty (Eds.), Advances in Pharmacology (Vol. 42, pp. 335–338). Academic Press. https://doi.org/10.1016/S1054-3589(08)60758-9
Compassionate Use of Stanate ™ [Stannsoporfin]. Retrieved October 26, 2017, from https://www.clinicaltrials.gov/ct2/show/NCT00076960?cond=neonatal+jaundice&draw=3&rank=15 default - Stanford Children’s Health. (n.d.). Retrieved October 25, 2017, from http://www.stanfordchildrens.org/en/topic/default?id=hyperbilirubinemia-and-jaundice-90-P02375
Chu, J. (2014). Approach to Jaundice in Infancy. Mount Sinai Expert Guides: Hepatology, 374-381.
Dennery, P. A., Seidman, D. S., & Stevenson, D. K. (2001). Neonatal Hyperbilirubinemia. New England Journal of Medicine, 344(8), 581–590. https://doi.org/10.1056/NEJM200102223440807
Glycerin Suppositories to Reduce Jaundice in Premature Infants- Study Results. Retrieved October 26, 2017 from https://www.clinicaltrials.gov/ct2/show/results/NCT01746511?sect=X9a70156&rslt=With&cond=neonatal+jaundice&rank=1#outcome3
Guillemette, C. (2003). Pharmacogenomics of human UDP-glucuronosyltransferase enzymes. The Pharmacogenomics Journal, 3(3), 136–158. https://doi.org/10.1038/sj.tpj.6500171
Hargreaves, T., & Piper, R. F. (1971). Breast milk jaundice. Archives of disease in childhood, 46(246), 195-198.
Kotal, P., Vitek, L., & Fevery, J. (1996). Fasting-related hyperbilirubinemia in rats: the effect of decreased intestinal motility. Gastroenterology, 111(1), 217-223.
Lightner, D. A., McDonagh, A. F. (1984). Molecular mechanisms of phototherapy for neonatal jaundice. Accts Chem Res; 17: 417-24.
Maisels, M. J. (2005). A primer on phototherapy for the jaundiced newborn: pediatricians need a keen understanding of the variables that influence how phototherapy lowers the bilirubin level and of how to deliver an optimal dose of light. Contemporary Pediatrics, 22(6), 38-46.
Maisels, M. J. (2006). Neonatal jaundice. Pediatrics in Review, 27(12), 443.
Maisels, M. J., & McDonagh, A. F. (2008). Phototherapy for neonatal jaundice. New England Journal of Medicine, 358(9), 920-928.
Marshall, W. J., Bangert, S. K., Lapsley, M. (2012). Clinical Chemistry Seventh Edition. Mobsy Ltd.
Martich-Kriss, V., Kollias, S. S., & Ball, W. S. (1995). MR findings in kernicterus. American journal of neuroradiology, 16(4), 819-821.
McDonagh, A. F. (2001). Phototherapy: from ancient Egypt to the new millennium. Journal of Perinatology, 21(S1), S7.
National Health Service. (2015, February 9). Jaundice. Retrieved October 26, 2017, from https://www.nhs.uk/Conditions/Jaundice/Pages/Introduction.aspx
National Institutes of Health. (2017, September 22). Urobilinogen in Urine: MedlinePlus Lab Test Information. Retrieved October 26, 2017, from https://medlineplus.gov/labtests/urobilinogeninurine.html
National Library of Medicine, G. H. R. (2017). UGT1A1 gene. Retrieved October 27, 2017, from https://ghr.nlm.nih.gov/gene/UGT1A1
Neonatal hyperbilirubinemia | McMaster Pathophysiology Review. (2015). Retrieved October 18, 2017, from http://www.pathophys.org/neonatal-hyperbilirubinemia/
Rennie, J., Burman-Roy, S., Murphy, M. S., & Guideline Development Group. (2010). Neonatal jaundice: summary of NICE guidance. BMJ, 340(7757), c2409.
Sarici, S. Ü., Serdar, M. A., Korkmaz, A., Erdem, G., Oran, O., Tekinalp, G., … & Yigit, S. (2004). Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics, 113(4), 775-780.
Setia, S., Villaveces, A., Dhillon, P., & Mueller, B. A. (2002). Neonatal jaundice in Asian, white, and mixed-race infants. Archives of pediatrics & adolescent medicine, 156(3), 276-279.
Steiner, L. A., Bizzarro, M. J., Ehrenkranz, R. A., & Gallagher, P. G. (2007). A decline in the frequency of neonatal exchange transfusions and its effect on exchange-related morbidity and mortality. Pediatrics, 120(1), 27-32.
Tan, K. L. (1982). The pattern of bilirubin response to phototherapy for neonatal hyperbilirubinemia. Pediatr Res; 16: 670-674.
Tan, K. L. (1998). Decreased response to phototherapy for neonatal jaundice in breast-fed infants. Archives of pediatrics & adolescent medicine, 152(12), 1187-1190.
Vreman, H. J., Wong, R. J., & Stevenson, D. K. (2004). Phototherapy: current methods and future directions. In Seminars in perinatology; 28(5): 326-333.
Wang, X., Chowdhury, J. R., & Chowdhury, N. R. (2006). Bilirubin metabolism: applied physiology. Current Paediatrics, 16(1), 70+. Retrieved from http://go.galegroup.com/ps/i.do?p=AONE&sw=w&u=tplmain&v=2.1&it=r&id=GALE%7CA143580890&sid=googleScholar&asid=413fc7b1d7f580273a5138d870068ea1
Woodgate, P., & Jardine, L. A. (2011). Neonatal jaundice. BMJ clinical evidence, 2011.