Table of Contents
What is Alzheimer's Disease?
Alzheimer's Disease is a type of neurodegenerative disorder that affects the physical, cognitive and behavioural functions of elderly individuals. Found by Dr.Alois Alzheimer', this disease comprises about 80 % of all the dementia cases worldwide. The symptoms for Alzheimer's Disease range anywhere from minor lapses in memory loss to severe cognitive declines with respect to carrying out everyday tasks. There are various theories that explain the causes and development of Alzheimer's disease, however. none are completely certain. There are also many approved drugs to alleviate the adverse symptoms of the disease, however, these drugs are not efficient in limiting its progression (Kumar & Singh, 2015). Although there is much progress in better understanding the overall disease as a whole, there is concern for the future as scientists are predicting that the overall cases of Alzheimer's disease will reach a staggering 24 million worldwide by 2040 (Reitz et al.,2011).
Symptoms
The most common symptom of Alzheimer’s diseases is difficulty remembering newly learned information (Sperling et al., 2011). Often times the diseases is noted by the family or friends because he or she does not realize they are suffering from the disease. Early signs of the disease are noted when the person has trouble recalling things that just happen, or putting thoughts into words (Sperling et al., 2011). As this disease progresses, symptoms will become more severe, leading to complications such as disorientation, mood and behaviour changes, and deepening confusion about events (Sperling et al., 2011).
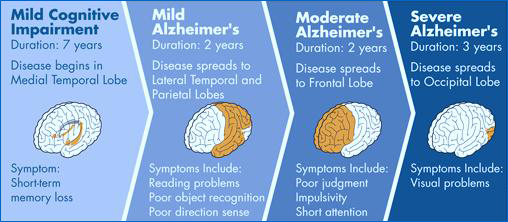
Alzheimer's disease is divided into 3 major stages. Please see Figure 1 for further details.The first stage of Alzheimer’s is categorized by the mild stage. This stage usually starts 2-4 years into the disease (Sperling et al., 2011). People that suffer from this stage have less energy and drive to do certain things. Their interest level dies down, and sleeping, sitting, watching TV becomes more attractive (Sperling et al., 2011). They have trouble recalling recent events. Also they have trouble putting thoughts into words. Coordination and movement can be hindered (Sperling et al., 2011). Trouble with driving and getting lost with familiar routes may occur. Though these are mild symptoms, they still have a huge impact on a person’s life. These symptoms can later lead to depression because of the lack of interest in things. More over these symptoms can be the result of other medical condition (Sperling et al., 2011). This is why it is important to consult a doctor when symptoms such as the one listed arise. The second sage is the moderate stage, were memory problems worsen compared to the mild stage. This stage lasts 2-10 years. In this stage details of one’s life are forgotten (Sperling et al., 2011). For example this can range from forgetting ones age, family members names, and family recognition. Furthermore rambling speech, hard time problem solving, and not dressing for the weather are complications that occur. Other symptoms are getting upset or happy at random times. These major mood swings can cause the lashing out on caregivers. Lastly trouble sleeping and wondering occurs. In this stage the people become more aware that they are losing control and frustration arises from this (Sperling et al., 2011). The last stage is the severe stage of Alzheimer’s, which typically lasts 1-3 years (Sperling et al., 2011). This stage comes with major confusion about past and current events. The person is not able to express or process information. Problems such as losing control of your bladder and bowels occur (Sperling et al., 2011). Furthermore extreme mood swings, hallucination, and extreme memory impairment all occur in this stage.
Figure 1: The stages of Alzheimer's disease starting from mild cognitive impairment leading up to more severe stages.
Prognosis
Before severe brain degeneration due to Alzheimer’s, it is crucial to diagnose this condition as early as possible. The disease can occur in patients as early as the age of forty (Mayeux & Sano, 1999). Usually these patients have inherited a mutant gene from their parents causing the dieses to show up earlier than usual (Mayeux & Sano, 1999). This is also known as the “early onset” (Mayeux & Sano, 1999). More commonly Alzheimer’s this dieses attacks individuals around there sixties (Mayeux & Sano, 1999). This form of Alzheimer’s is called the “Late onset”. Studies show that over 90% of individuals are effected around this age (Mayeux & Sano, 1999). However the Late set-onset of Alzheimer’s disease is not completely understood as of yet. Studies show that a mix of environmental, genetic, and life style factors may play a role (Mayeux & Sano, 1999). Both the early set and late set have similar patterns of damage showing up in the brain. Moreover the increasing of one’s age shows a correlation with attaining the dieses (Mayeux & Sano, 1999). Though this is the case the symptoms and signs of Alzheimer’s and dementia are not part of normal aging.
Epidemiology
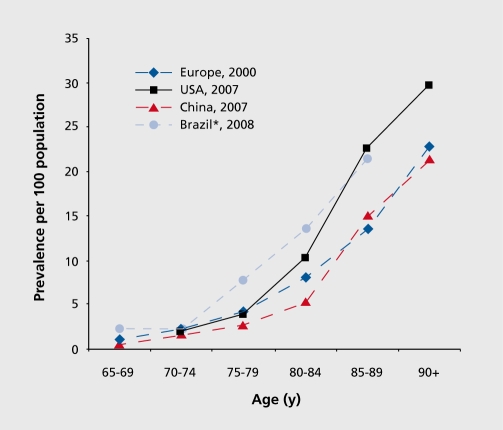
Globally there is an estimated 24 million people with Alzheimer’s disease with 4.6 million cases rising every year (Reitz, Brayne & Mayeux, 2011). This number is predicted to double every 20 years. More over as the population of North America is aging, thus the number of patients with the disorder are increasing particularly in the elderly community (Reitz et al., 2011). The highest prevalence of Alzheimer’s is shown in North America, Western Europe, Latin America and China (Reitz et al., 2011).The age at which people are affected by the disorder ranges around the age of 60 (Reitz et al., 2011). The annual incident rates (per 1000) in these countries are 10.5 in North America, 8.8 in Western Europe, 9.2 in Latin America and 8.0 in China (Reitz et al., 2011). Moreover the prevalence rates of Alzheimer’s disease rise exponentially with age (Reitz et al., 2011). Compared to Europe china and Latin America the prevalence of Alzheimer’s is mainly in the United States (Reitz et al., 2011). The prevalence seems to be higher in the Latin American and African American communities. But the rates are much lower for African communities in their homeland. The reason for this still remains unsure. By the year 2020 World health
Organization’s predict the number of Alzheimer’s patients to reach 29 million (Reitz et al., 2011). Risk factors such as family history and genetic factors can play a huge role in developing Alzheimer’s disease. Individuals with Parents, brothers or sisters with the disease are more likely to develop the disorder. There is an increase in the risk of attaining the illness if more than one family member has the disease. Furthermore scientist have discovered risk genes that increase the likelihood of someone developing the disease. The gene with the strongest risk factor is called the apolipoprotein E-e4 (APOE-e4) which is a factor that increases the chances of Alzheimer’s by 20-25 % (Reitz et al., 2011). If you inherit one APOE-e4 gene from a parent you will have an increased risk, but if you inherit from both parents have an even higher risk (Reitz et al., 2011). By inheriting the gene from both parents the signs and symptoms of Alzheimer’s is going to show up at a younger age than usual (Reitz et al., 2011). Moreover genes that guarantee the disorder are called deterministic genes (Reitz et al., 2011). By coding three proteins, scientists have discovered the genes the variations that directly cause Alzheimer’s disease (Reitz et al., 2011). The three proteins are amyloid precursor protein (APP), presenilin-1 (PS-1) and presenilin-2(PS-2) (Reitz et al., 2011). When these genes cause the Alzheimer’s disorder, it is called “autosomal dominant Alzheimer’s disease (ADAD) or Familial Alzheimer’s disease (Reitz et al., 2011). When effected by this autosomal disease the symptoms usually appear well before the age of 60. It may occur as early as a person’s 30s or 40s. This form of Alzheimer’s have been found in only a few hundred families worldwide (Reitz et al., 2011). This rare form of Alzheimer’s accounts for less than 5% of cases worldwide (Reitz et al., 2011).
Figure 2: This graph depicts the prevalence of the disease as being most abundant in Brazil, Europe, USA and China.
Pathophysiology
Neuropathology
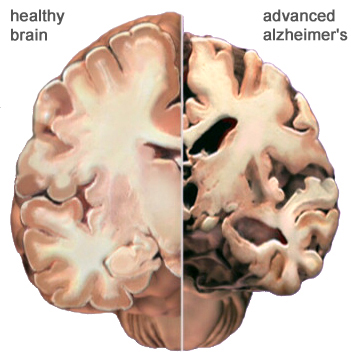
The hallmarks of Alzheimer’s disease involve a loss of neurons, the shrinkage of large cortical neurons and synapses in the brain over a period of time. The result is an eventual degeneration in the cingulate gyrus, temporal and parietal lobes, the frontal cortex, brainstem nuclei and locus coruleus. In addition, the disease is characterized amyloid plaques and neurofibrillary tangles concentrated in vulnerable areas in the brain and are visible under a microscope. The plaques are made up fibrous proteins known as β-amyloid, diffuse plaques (poorly defined amyloids) and burnt-out plaques containing an isolated amyloid core. These plaques form clumps in the cortex and are hypothesized to result in a cascade of events leading to cell dysfunction and necrosis. (Yaari, 2007) Even with increasing evidence to suggest this, it is not a view supported by all. (Hardy, 1991). Neurofibrillary tangles are associated with tau, a microtubule protein that has become hyperphosphorylated and clustered together with others of its kind. Though these plaques and tangles are not only seen Alzheimer’s sufferers, those who do suffer from the disease exhibit a much larger prevalence of them than one who does not suffer from it. (Bouras, 1994).
In Alzheimer’s disease, polymerization and misfolding of generally soluble proteins occurs as a result of the accumulation of the plaques inside cells as discussed above. (Selkoe, 2004) The plaques are composed of the peptides β-amyloid (part of the amyloid precursor protein), sized 39-43 amino acids long. The precursor protein’s cell function in the brain is associated with the growth and survival of neurons. (Yaari, 2007) When phosphorylated, the protein tau stabilizes microtubules. In Alzheimer’s sufferers hyperphosphorylation occurs in the tau proteins, making it aggregate much more easily to form neurofibrillary tangles. This essentially disengages the transport system of the neurons. (Hernandez, 2007)
Figure 3:This figure shows a comparison of a normal brain and one with Alzheimer's disease. The atrophy and degeneration of brain tissue in the brain on the right is visible. (Alz, 2011).
Disease Mechanism
The biochemistry or course of actions that results in Alzheimer’s disease is not yet definite. Many hypotheses have been established over the years in order to explain the phenomenon. Of all of the existing theories, three have gained ample recognition and support in comparison to the rest. Before examining the theories below, it is important to note that there is no causational link clearly identified in the literature. The three most popular hypotheses include: Amyloid Hypothesis, Cholinergic hypothesis and Tau hypothesis.
Amyloid Hypothesis
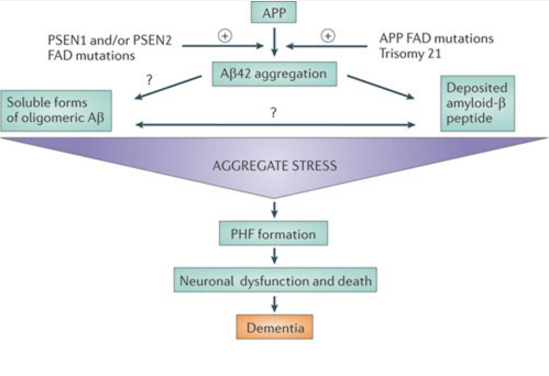
The foundation of this theory rests upon the idea that aggregated β-amyloid fibers lead to cytotoxicity within cells and in turn disrupt calcium ion homeostasis, leading to apoptosis. This apoptosis leads to the death of brain matter as seen in the brains of Alzheimer’s sufferers. (Yanker, 1990) Several mutations have been linked to early onset familial Alzheimer’s disease in three genes: PSEN1, PSEN2, and amyloid precursor protein APP. Such mutations would cause aggregate stress and disrupt the normal function of β-amyloid peptide, leading to aggregation. (Karran, 2011) In a study conducted by Lijima et. Al (2004), mouse models showed support for the theory as they demonstrated that β-amyloid aggregates caused them cognitive impairment. Another reason this theory has gained popularity is due to chromosomal effects. the APP gene is located on chromosome 21, and those with trisomy 21 (down syndrome) exhibit similar late cognitive effects as those with Alzheimer’s. (Nistor, 2007)
Figure 4:This figure shows a simplification of the cascade of events that occur in Alzheimer's disease as predicted by the Amyloid Hypothesis. (Karran, 2011).
Cholinergic Hypothesis
One of the older theories, the cholinergic hypothesis states that Alzheimer’s associated brain tissue degradation is a result of the deficiency in the neurotransmitter acetylcholine. Research now shows that there isn’t a causational relationship acetylcholine levels and the onset of Alzheimer’s disease. It is shown, however, that lower levels of acetylcholine are present in patients with the disease and this may be due to brain tissue damage. In the recent past, the neurotransmitter has been proposed to be a potential causal agent in the formation of aggregates and neuroinflammation. (Shen, 2004)
Tau Hypothesis
The Tau Hypothesis suggests that the formation of Neurofibrillary tangles (NFTs) –that is characteristic of Alzheimer’s Disease– is due to the hyperphosphorylation of Tau proteins. Tau proteins normally play a role of structural support as they are responsible for microtubule stabilization. However, Tau Hyphosphorylation results in clumping of other Tau proteins which eventually develop into NFTs in Alzheimer’s Disease. Research has shown that Tau Hyperphosphorylation has led to neuronal cell death through microtubule dysfunction which disrupts the structural integrity of the cell. As a result, this has been associated with the cognitive decline that is seen in Alzheimer’s Disease (Maccioni et al., 2010).
Intervention Strategies
Treatment
The treatment of Alzheimer’s disease primarily involves the use of pharmacological measures to alleviate the adverse symptoms that patients experience. One prominent pharmacological treatment is the administration of Cholinesterase inhibitors. The use of these drugs has shown great promise in clinical trials with respect to the treatment of AD physical symptoms and it has shown to even improve cognitive performance in individuals with AD. These drugs inhibit the enzyme, Acetylcholinesterase in the neuronal synapse which leads to a greater concentration and increased temporal activation of the neurotransmitter, Acetylcholinesterase. There are four commonly used drugs that act as Cholinesterase inhibitors and they are Tacrine, Donepezil, Rivastigmine, and Galantamine, respectively (Grossberg & Desai, 2003). Other pharmacological drugs include the administration of antioxidants such as Alpha tocopherol and selegiline. These compounds function by inhibiting an enzyme that is involved in oxidative stress in neuronal cells. Antioxidants have also shown to be promising in the treatment of AD as there have been clinical trials that demonstrated a slower rate of AD development in groups that were given selegiline (Ibbotson & Goa, 2002).
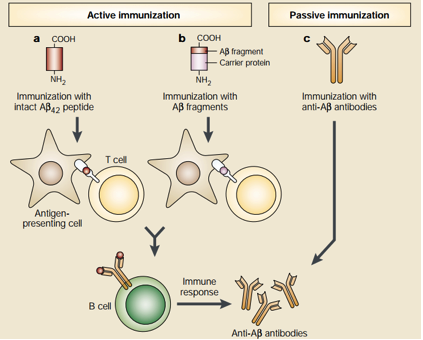
Even though antioxidants and Cholinesterase inhibitors are effective in treating AD symptoms, they are not effective in altering the overall pathogenesis of AD. For these reasons, novel treatments are been developed—especially by targeting Amyloid-β plaques through the form of Amyloid-β immunotherapy. This unique form of treatment utilizes the immune system to stop the accumulation of Amyloid-β plaques. Amyloid-β immunotherapy is divided into two different types which are active and passive immunotherapy, respectively. See Figure below for details. Active immunization is further divided into two types. The first type involves the injection of the 42 amino acid Aβ peptide which subsequently results in Antigen Presenting Cell (APC) recognition and phagocytosis of these peptides. The APCs then present the processed Aβ peptide to T cells which leads to the proliferation of B cells that produce anti-Aβ plaque antibodies that are specific for the Aβ peptide. The second active immunization type uses almost the same principle as the first type but differs in the initial administration. Instead of direct administration of the Aβ peptide, small fragments of the Aβ peptide are attached to a carrier protein and the carrier protein is the antigen that is recognized by APCs. On the other hand, passive immunization involves direct administration of anti-Aβ plaque antibodies and this would be useful for individuals who are unable to generate an immune response to the Aβ plaques. It is important to note that Aβ immunotherapy has shown promising results in mice models but further research needs to be conduced in humans for effective AD treatment (Schenk, 2002). Please see Figure 4 for details.
Figure 5: This figure shows two promising approaches of utilizing anti-Aβ plaque antibodies to limit the progression of Alzheimer's Disease (Schenk, 2002).
Management
The management of Alzheimer’s disease involves the contributions of a variety of health care professionals to control the severity of the patient’s symptoms. To begin, physicians are crucial in the management of AD as they are the primary means to recommend pharmacological treatments for treating symptoms. Physicians treat various physical symptoms that are associated with AD such as insomnia and aggression. Clinical psychologists are also crucial for the treatment of behavioral aspects of the disease such as depression and anxiety. Lastly, social workers are responsible for providing emotional and physical support to the patient and the family members (Grossberg & Desai, 2003).
Alzheimer’s disease can also be managed through therapeutic measures, particularly non-pharmacological means. One type includes psychosocial management which includes educating the patient’s caregivers to reduce the distress of both the caregivers and the patient. Caregivers can also be trained in a management technique called Interpersonal management which involves attending to patient’s unmet needs such as pain, hunger and thirst. Finally, environmental management techniques are important for treating AD patients who suffer from neurological symptoms as a result of changing environmental stimulations. Specifically, a condition that AD patients frequently experience is termed the sundowning phenomenon, which is when patients experience greater distress and neurological symptoms during daytime periods of high sun time. To reduce these symptoms, it is advised to make patients feel comfortable through various relaxation techniques (Gauthier et al., 2010).
Prevention
Although Alzheimer's Disease (AD) has a number of non-modifiable genetic risk factors, there are also potentially modifiable risk factors such as hypertension, hyperlipidemia and environmental exposures that can be improved with improved with proper care and preventative measures (Patterson et al., 2008). Beginning with genetic factors, there are three gene mutations that almost always result in the occurrence of Alzheimer's Disease. These include a mutation in either the amyloid precursor protein gene, presenilin-1 gene or presenilin-2 gene (Patterson et al., 2008). In terms of non-genetic factors, studies have shown a correlation between systolic blood pressure and an increased risk of dementia. After the study group with high systolic blood pressure received antihypertensive treatment, the incidence of dementia was reduced by 50% from 7.7 to 3.8 cases per 1000 patients in the treatment group (Patterson et al., 2008). A second non-genetic risk factor for Alzheimer's is the increased risk of the disease with hyperlipidemia. Speculative studies have suggested that increased levels of total serum cholesterol levels have been linked to increased risk of Alzheimer's (Patterson et al., 2008). However, there are many studies that have downplayed the role of increased cholesterol in Alzheimer's patients. For instance, there is no correlation between cholesterol and beta-amyloid peptide production (Ledesma et al., 2005). Despite the many factors leading the Alzheimer's, the consumption of several medications have been known to reduce the risk of the disease. These primarily include non-steroidal anti-inflammatory drugs (NSAIDs) and vitamin supplements (Patterson et al., 2008). Follow-up studies revealed that although the consumption of NSAIDs does not directly reduce the risk of Alzheimer's, patients who took an NSAID such as Naproxen were protected from the onset of AD by 67% compared to the placebo (Imbimbo, 2010). More specifically, it has been hypothesized that the consumption of NSAIDs might only be beneficial in the early stages of AD, during the initial Beta-Amyloid deposition process (Imbimbo, 2010).
Further Research and Future Applications
The current issue with the treatments available for Alzheimer's today are due to the fact that only symptomatic treatments exist, which all try to counteract the neurotransmitter imbalance (Yiannopoulou et al., 2013). Still under extensive research are the “disease-modifying drugs” which alter the course of the disease. In order to stop the onset of Alzheimer's, future drugs must be able to disturb the pathogenic steps responsible for the symptoms. Some of these steps include the deposition of Beta-Amyloid plaques, inflammation, oxidative damage and iron deregulation (Yiannopoulou et al., 2013). Current disease-modifying therapies for AD are in phase I-III of their trials. All the future research will be heavily focused on preventing the deposition and misfolding of Beta-Amyloid and tau proteins. Prospective drugs as of right now include tramiprosate, currently in phase III of clinical trials and colostrinin which is currently in phase II (Yiannopoulou, 2013). Tramiprosate aims to interfere with the binding of glycosaminoglycans and beta-amyloid while colostrinin inhibits beta-amyloid aggression and improves cognitive performance in animal models (Yiannopoulou, 2013). Another possible strategy under study is immunotherapy. Both active immunization through vaccination and passive immunization through monoclonal antibodies are being studies (Yiannopoulou, 2013). This form of treatment proved to be controversial after a phase II vaccination trial resulted in cases of meningoencephalitis in 6% of the cases.
Conclusion
To summarize, Alzheimer's Disease is a very common case of dementia that has significant implications the future health and well being of elderly patients. Currently, the most accepted hypothesis by scientists is the Beta-amyloid hypothesis which describes possible processing faults involved with the Amyloid Precursor Protein (APP) causing degradation of brain tissue. In most cases, epidemiology shows that age is a main factor for Alzheimer's disease to appear. Characterized by a loss of cognitive function, this debilitating disease unfortunately has no forms of prevention other than eating foods that boost cognitive functions and brain activity. In terms of future research, a novel treatment that has recently been discovered is the use of immunization to stop the development of the disease. Through techniques of active or passive immunization, the administration of antibodies causes alterations and improvement in the beta-amyloid plaques. With such a high onset rate at relatively high ages, future scientific research will be essential to continue to find new preventative measures and more effective treatments.
References
(“Healthy Brain Versus Alzheimer's Brain | Alzheimer's Association,” 2011)
A next-generation approach to Alzheimer's treatment. (n.d.). Retrieved April 01, 2016, from http://www.cobas.com/home/innovation/next-gen-alzheimers-treatment.html
(Bouras, Hof, Giannakopoulos, Michel, & Morrison, “Regional Distribution of Neurofibrillary Tangles and Senile Plaques in the Cerebral Cortex of Elderly Patients: A Quantitative Evaluation of a One-Year Autopsy Population from a Geriatric Hospital,” 1994, pp. 138-150)
(Hardy & Allsop, “Amyloid deposition as the central event in the aetiology of Alzheimer's disease,” 1991, pp. 383-388)
Gauthier, S., Cummings, J., Ballard, C., Brodaty, H., Grossberg, G., Robert, P., & Lyketsos, C. (2010). Management of behavioral problems in Alzheimer's disease. International Psychogeriatrics, 22(03), 346-372.
Grossberg, G. T., & Desai, A. K. (2003). Management of Alzheimer's disease. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 58(4), M331-M353. Management
Hernández, F., & Avila, J. (2007). Tauopathies. Cell. Mol. Life Sci. Cellular and Molecular Life Sciences,64(17), 2219-2233.
Ibbotson, T., & Goa, K. L. (2002). Management of Alzheimer’s Disease. Disease management and health outcomes, 10(1), 41-54.
Iijima, K., Liu, H., Chiang, A., Hearn, S. A., Konsolaki, M., & Zhong, Y. (2004). Dissecting the pathological effects of human A 40 and A 42 in Drosophila: A potential model for Alzheimer's disease. Proceedings of the National Academy of Sciences, 101(17), 6623-6628.
Imbimbo, B.P., Solfrizzi, V., & Panza, F. (2010). Are NSAIDs Useful to Treat Alzheimer's Disease or Mild Cognitive Impairment?Frontiers in Aging Neuroscience, (2), 19
Karran, E., Mercken, M., & Strooper, B. D. (2011). The amyloid cascade hypothesis for Alzheimer's disease: An appraisal for the development of therapeutics. Nature Reviews Drug Discovery Nat Rev Drug Discov,10(9), 698-712.
Kumar, A., & Singh, A. (2015). A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacological Reports, 67(2), 195-203.
Ledesma, M.D., & Dotti, G.C. (2005). The conflicting role of brain cholesterol in Alzheimer's disease: lessons from the brain plasminogen system. PubMed, (72), 129-138
Maccioni, R. B., Farías, G., Morales, I., & Navarrete, L. (2010). The revitalized tau hypothesis on Alzheimer's disease. Archives of medical research, 41(3), 226-231.
Mayeux, R., & Sano, M. (1999). Treatment of Alzheimer’s disease. N Engl J Med, 341(22), 1670-9.
Nistor, M., Don, M., Parekh, M., Sarsoza, F., Goodus, M., Lopez, G., . . . Head, E. (2007). Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiology of Aging, 28(10), 1493-1506.
Patterson, C., Feightner, J.W., Garcia, A., Hsiung, R., MacKnight, C., & Sadovnick, A.D. (2008). Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Practice, 178(5), 548-556.
Reitz, C., Brayne, C., & Mayeux, R. (2011). Epidemiology of Alzheimer disease. Nature Reviews Neurology, 7(3), 137-152.
Schenk, D. (2002). Amyloid-β immunotherapy for Alzheimer's disease: the end of the beginning. Nature Reviews Neuroscience, 3(10), 824-828.
Shen, Z. (2004). Brain cholinesterases: II. The molecular and cellular basis of Alzheimer's disease. Medical Hypotheses, 63(2), 308-321.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., … & Park, D. C. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 280-292.
Yankner, B., Duffy, L., & Kirschner, D. (1990). Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science, 250(4978), 279-282.
Yiannopoulou, K.G., & Papageorgiou, S.G. (2013). Current and future treatments for Alzheimer's disease. Therapeutic Advances in Neurological Disorders, 6(1), 19-33