Table of Contents
Love and Hate
Love is comprised of various positive and strong emotional experiences and mental states. Driving love are three components: lust, attachment, and attraction. Each component of love is influenced by various hormones and neurotransmitters that affect our brain's reward circuit. Two regions that become active when in love include the caudate nucleus and the ventral tegmental area, also known as the reward circuit. This circuit is linked with the nucleus accumbens, with the function of operating dopamine and serotonin. Other structures that contribute to the reward circuit include the amygdala, the hippocampus, and the prefrontal cortex. In contrast, heartbreaks result from traumatic breakups or the death of a loved one. In extreme events the emotional distress can be so severe it can result in the alteration of the left ventricle. As a consequence, many symptoms arise and are known to be associated with the heartbreak syndrome, also known as Takotsubo Cardiomyopathy (TC). Neurochemical processes such a the μ-opioid receptor and drastic declines in love neurotransmitters such as dopamine and oxytocin have been shown to cause the brain to react negatively and seek out social contact. Beyond heartbreak, hate is also a common emotion that is felt. Hate, in its simplest form, is to have a strong emotional response to extreme dislike. One can manifest a hatred for a variety of things, including an individual and certain groups of people. A study based on “the hate circuit” has proposed that the reason why we hate is due to the same four areas of the brain being activated, which is consistent across all individuals and which help with motor and decision planning when confronted with a hated person. Potential treatments include painkillers, opioids, placebo treatments, social support and even comfort food to demission activation of areas of the brain that cause both physical pain and emotional pain.
What is Love?
Love pertains to multiple strong and positively experienced mental and emotional states, ranging from deep affection to simple pleasures (Love, 2018).
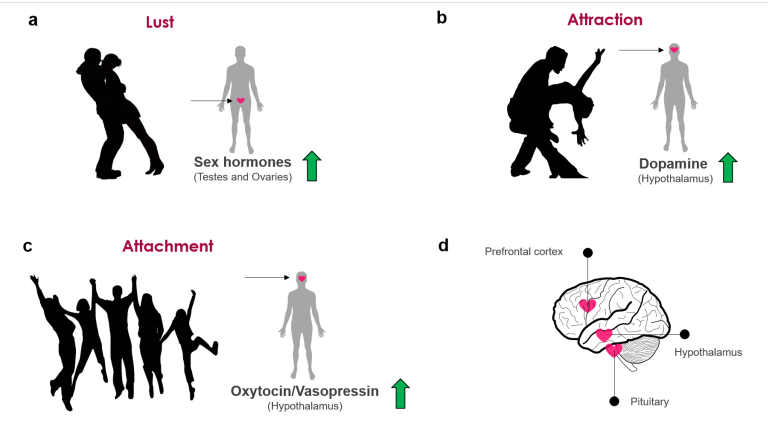
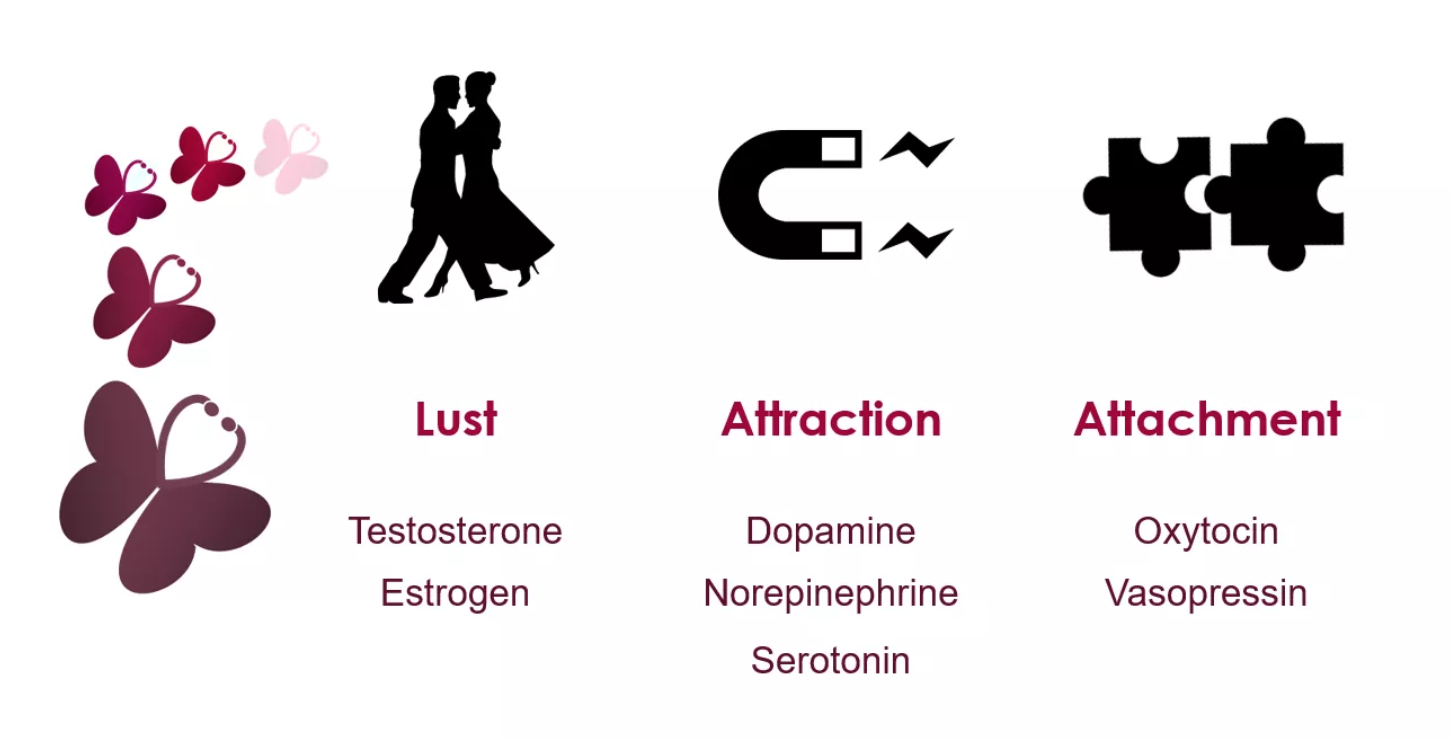
So why do we fall in love? It is thought that the romance part of love is one of three basic brains systems that evolved for mating and production. There are three components to love: sex drive or lust, romantic love or attraction, and finally attachment (Fisher H, 2018).
- Sex drive or lust: The desire for sexual gratification, allowing an individual to seek a range of potential mating partners. It's important to note, you do not have to be in love to experience lust (Fisher H, 2018).
- Romantic love, or attraction: Obsessive thinking or craving for an individual. It's been suggested that this process evolved to allow individuals to focus their mating energy on one person (Fisher H, 2018).
- Attachment: A feeling of deep union and connection with an individual, evolved to ensure that you remain with your mate long enough to ensure the survival of your offspring (Fisher H, 2018).
Biology of Love
It is known that lust is driven by sexual gratification. This need to be fulfilled sexually is driven by the hypothalamus, which signals to the ovaries and testes to release the hormones estrogen & testosterone (Wu, 2018). Both hormones impact the male & female sexually, where testosterone is thought to increase one’s sexual libido (Wu, 2018). Contrast to this is estrogen which is thought to have a lesser effect than testosterone. Despite this, some females have indicated feeling the most sexually motivated around ovulation when estrogen levels are highest.
Typically, one lusts for one they are attracted to and vice versa. However, it is possible to experience one and not the other. When you're attracted to someone the brain pathways that influence reward behaviour becomes activated. The activation of this pathway can explain why the first few months of being with someone can feel almost euphoric. The activation of this pathway affects the release of norepinephrine, dopamine & serotonin (Wu, 2018). Dopamine is released from the hypothalamus when we do things that make us feel good in this case spending time or engaging in intercourse with are mate. As dopamine levels peak, norepinephrine is released which is responsible for our high energy and euphoria during attraction, it can also cause loss of appetite and sleep. As these neurotransmitters increase, serotonin begins to decrease. It has been speculated that this decrease in serotonin is related to the overpowering infatuation that occurs with the beginning of love (Wu, 2018).
Attachment is what mediates long term relationships, whether this is romantic, friendship or family related. The hormones responsible for attachment are oxytocin & vasopressin. Similar to dopamine, oxytocin is produced by the hypothalamus (Wu, 2018). Large amounts of oxytocin are then released during sex helping to form feelings of bonds and romantic attachments (Wu, 2018). While it is not completely understood, it thought that increased levels of vasopressin are what allows for monogamy to occur. In a study done on moles that are typically promiscuous, the addition of the vasopressin receptor leads to them being pursuing only one female (Wu, 2018).
It has been suggested, that being in love acts in a feedback loop manner where the brains reward system is activated. The feedback loop is affected by the central nervous system and levels of neurotransmitters found in the blood (Fisher M, 2018). The activated system causes neurotransmitters to be released to various parts of the body like the skin, genitals, and stomach, when stimulated they send messages back to the brain. For example, if stimulation of the genitals feels good, this information is sent back to the brain where the system is activated and the individuals seek out more stimulation (Fisher M, 2018).
The Brain on Love
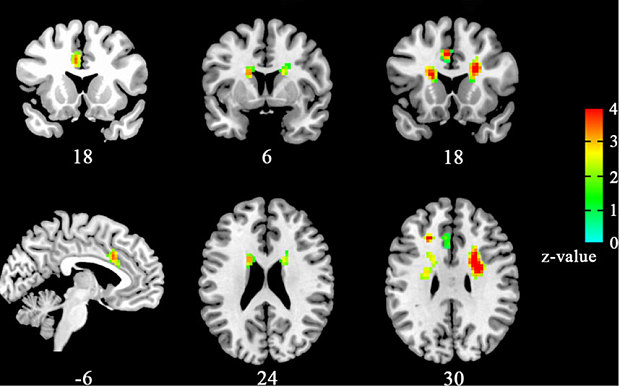
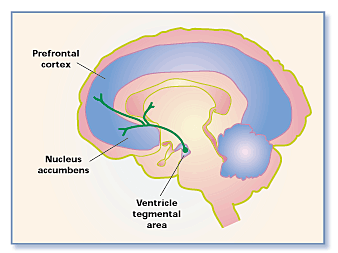
There have been studies demonstrating functional MRI (fMRI) images of the brain of individuals when they were shown photos of people they romantically loved. As a result, the brain became active in regions rich with dopamine which is known as the “feel-good neurotransmitter” (Fisher 2005). Specifically, two regions became active including the caudate nucleus, associated with reward detection and integration of sensory experiences into social behavior, and the ventral tegmental area, also known as the reward circuit is associated with pleasure and focused attention and motivation to acquire rewards (Fisher 2005). Rewards are defined as the biological mechanisms that motivate behavior by events associated with pleasure.
This reward circuit is considered to be a primitive neural network, therefore is evolutionarily old (Fisher 2005). It is linked with the nucleus accumbens, with the function of operating dopamine (promoting desire) and serotonin (satiety and inhibition). Other structures that contribute to the reward circuit include the amygdala, the hippocampus, and the prefrontal cortex (Fisher 2005). These are very sensitive to behavior associated with pleasure, such as sex, food consumption, and drug use. Chemicals associated with the reward circuit flood our brain, producing a variety of physical and emotional responses when we are falling in love. The responses include a racing heartbeat, sweaty palms, flushed cheeks, feelings of passion and anxiety (Fisher 2005). In addition to this, levels of the stress hormone cortisol increase during the initial phase of romantic love. As cortisol levels rise, levels of the neurotransmitter serotonin become depleted (Fisher 2005). Finally, adrenaline and norepinephrine are responsible for the increased beating of the heart, restlessness and overall preoccupation that go along with experiencing love (LUHS, 2014).
Biochemistry of Love
Dopamine activates the reward circuit, making love a pleasurable experience similar to the euphoria, associated with drugs and alcohol (Fisher 2005). A Study from the University of California demonstrated that male fruit flies that were sexually rejected drank four times as much alcohol as fruit flies that mated with female fruit flies (Fisher 2005). Other chemicals shown during romantic love are oxytocin and vasopressin, that are hormones that play a role in pregnancy, nursing, and mother-infant attachment (Carter, 2012). Oxytocin is released during sex and is enhanced during skin to skin contact. It is the deepened feelings of attachment and makes couples feel closer to one another after having sex (Carter, 2012).This hormone is known as the love hormone that promotes feelings of contentment, calmness, and security, often associated with mate bonding (Carter, 2012). Vasopressin is linked with behaviours that produce long-term relationships. The differences in behaviour associated with the two hormones explain why passionate love fades as attachment grows (Carter, 2012).
What is Heartbreak?
A heartbreak can feel like the absolute worst feeling in the entire world. It can cause intense pain and emotional distress and even make you believe you don't want to live anymore. It can happen when a relationship has come to an end or you have lost a loved one, or simply from disappointment, betrayal. It can be caused by many things.
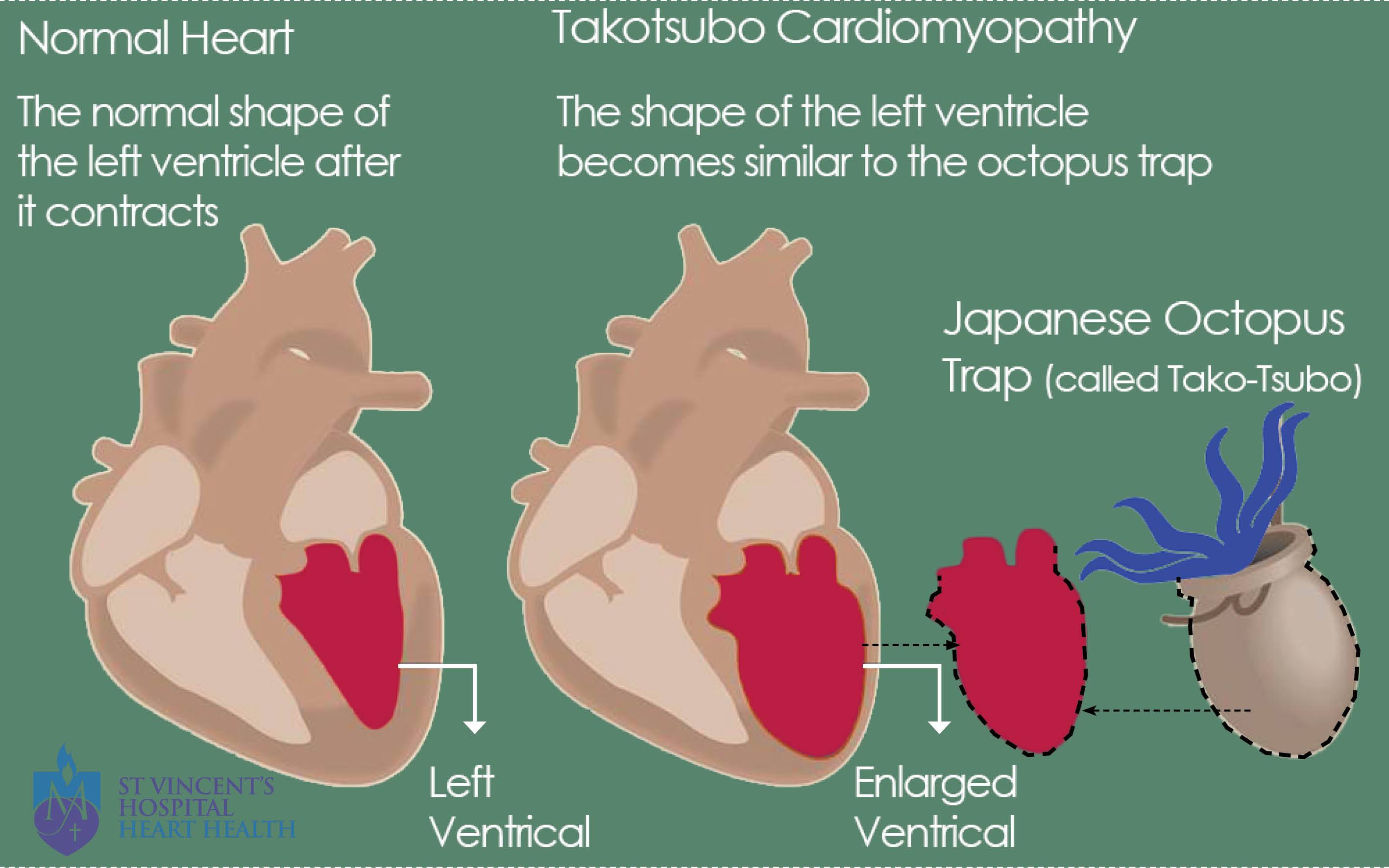
A heartbreak or heartbreak syndrome is also referred to as Takotsubo cardiomyopathy (TC). It is defined as the weakening of the muscular portion of your heart that is triggered by emotional stress. Emotional pain caused by a traumatic breakup or the death of a loved one can become so severe that it could actually damage your heart (Takotsubo cardiomyopathy, 2010). Heartbreak can mimic symptoms of a heart attack, but clinically they differ as arteries are not blocked and usually do not have the risk factors for heart disease. However, the symptoms are similar. Symptoms include chest pain, shortness of breath and sweating (Takotsubo cardiomyopathy, 2010). A research study conducted discovered that 6837 patients were diagnosed with the disorder among 33, 506, 402 hospitalizations in the Nationwide Inpatient Sample database. In other words, TC accounted for about 0.02% of all the hospitalizations in the United States (Deshmukh et al., 2012). It was discovered that women over the age of 55 years old had 4.8 times higher the probability of developing the TC disorder. However, other factors such as smoking, alcohol abuse, anxiety states, and hyperlipidemia also contributed to TC. Research on larger samples have not been fully conducted as the syndrome is relevantly new as it was discovered only about 20 years ago (Deshmukh et al., 2012).
Physiology of Heartbreak
Takotsubo cardiomyopathy is the weakening of the left ventricle, which is the heart's main pumping chamber. This is usually caused by severe emotional or physical stress. The precise cause of this syndrome is unknown but experts think that surging stress hormones like adrenaline essentially “stun” the heart, triggering changes in heart muscle cells or coronary blood vessels or even both that prevent the left ventricle from contracting effectively (Fisher, Xu, Aron & Brown, 2016). Other hormone levels like norepinephrine and epinephrine also are elevated in individuals with broken heart syndrome. The echocardiograms of individuals with broken heart syndrome usually have a weakened contraction in the middle and upper portions of the heart muscle, and inverted T waves and prolonged Q-T intervals often associated with stress (Fisher, Xu, Aron & Brown, 2016). Heartbreaks can also jeopardize one’s health due to abandonment rage as it stresses the heart consequently raising blood pressure and suppressing the immune system. This can further induce clinical depression and in extreme cases even lead to suicide (Fisher, Xu, Aron & Brown, 2016).
The Brain on Heartbreak
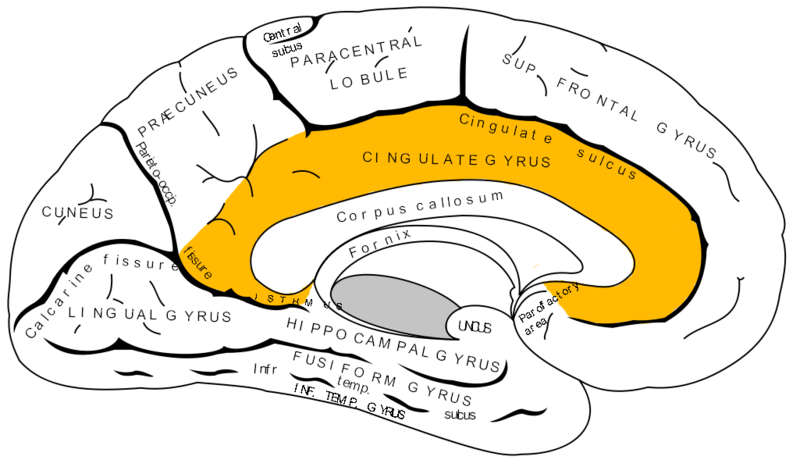
The neurological process involved in the perception of heartache is not known but is thought to involve the anterior cingulate cortex of the brain, which during stress may overstimulate the vagus nerve causing pain, nausea or muscle tightness in the chest.
Research published by the National Academy of Sciences showed that the pain we experience in moments like heartbreak is actually quite literal: our brains and bodies process the pain of emotional rejection in much the same way they do a sprained ankle or a broken leg (Vastag, 2003). Research participants viewed a photo of an ex after an unwanted breakup with that person and were told to think about the rejection of that breakup (Vastag, 2003). As they did, under neuroimaging the same regions of the brain that underpin the response to physical pain became active. Revealing that emotional pain such as social rejection and physical pain are similar as they share a common representation in somatosensory brain systems (Vastag, 2003). In the case of physical injury, there is first an adrenaline surge of stress hormones that prepare the body for flight or fight causing the individual to feel numb before the hormone surge dissipation and the pain reaction sets in. Researchers found the same reaction to occur after social rejection as well (Vastag, 2003).
Biochemistry of Heartbreak
Reduced Neurochemical Activity
As discussed previously, feelings of love and adoration have been observed to elevate certain neurochemicals, in particular, dopamine activity in the reward pathway and oxytocin which has also been both associated with feelings of trust and social connection in humans (Burnett, 2016). Over a period of time, the brain soon becomes accustomed to these elevated levels positive neurochemicals, however, when they unexpectedly end due to cases such as heartbreak, the brain turns to a state of shock (Burnett, 2016). All the positive neurochemicals the brain has come to expect due to being in a relationship and having them all drastically decline after a relationship ceases can negatively affect the brain by inducing this state of distress, uncertainty, and ambiguity (Burnett, 2016). Multiple studies have also shown that these sudden neurochemical decreases due to relationship breakups have also demonstrated to activate similar brain regions that process physical pain (Burnett, 2016).
μ-Opioid Receptor
Another mechanism that has been found to modulate heartbreak is the endogenous opioid system, a primary neurochemical system for regulating physical pain, as demonstrated in its ability to mediate social attachments in various animal and human studies (Tchalova & Eisenberger, 2015). In particular, it is proposed that social separation distress, much like the distress defined in heartbreak, can cause a painful low-opioid state. This low-opioid state can then motivate social proximity seeking, in other words, motivation to establish relationships/connections with other individuals (Tchalova & Eisenberger, 2015). This seeking behaviour ends once social contact is made, prompting the release of endogenous opioids and establishing regular opioid levels once again (Tchalova & Eisenberger, 2015).
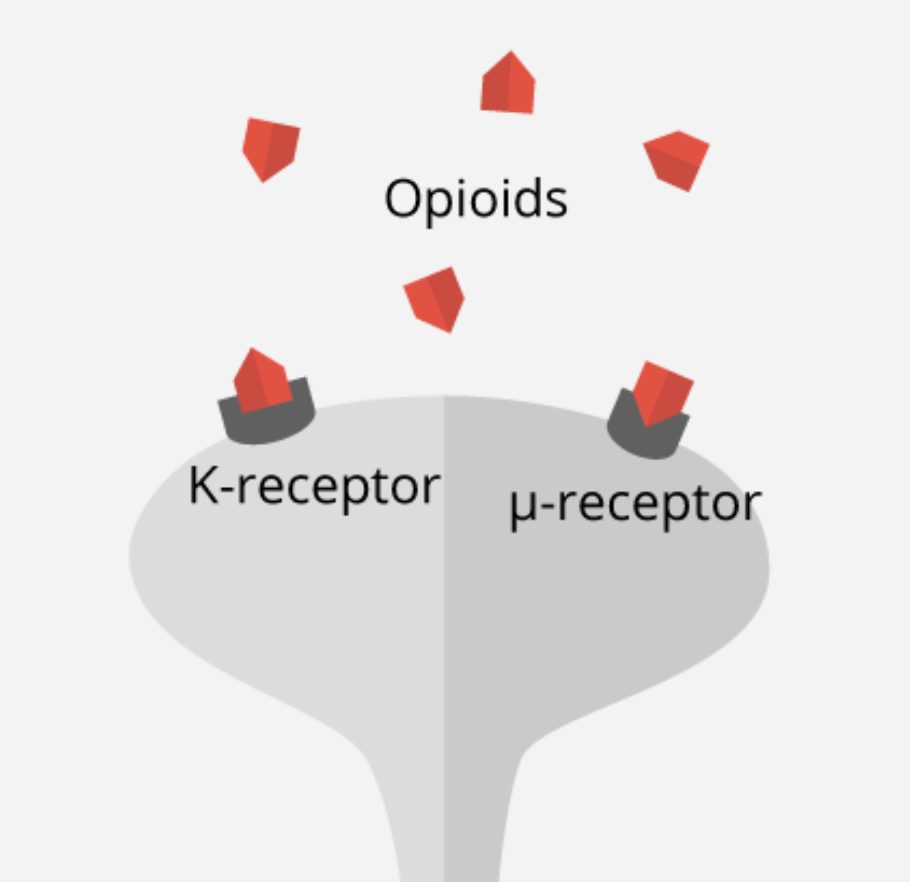
To examine this phenomenon further, studies have been conducted to examine μ-opioid, which has been known to be released as an emotional and physical painkiller during both types of events; when someone feels physical pain, as well as when a person experiences rejection and social detachment (Fisher, 2015). Moles and colleagues (2004) conducted a study on the μ-opioid receptor, which attaches to opioid molecules to complete the endogenous opioid system and plays a key role in pain perception and addiction. In this study, μ-opioid receptors (Figure 8) were genetically engineered and removed in mice models. The removal of this specific receptor resulted in an induced increase of distress vocalizations when mice pups were removed from their mother (Moles et al., 2004). Results from this study have thus helped indicate an associated molecular mechanism for abnormalities and deficits in social attachment behavior, as well as experiences of social distress (Moles et al., 2004).
The mutation of the μ-opioid receptor gene, specifically A118G polymorphism, has also been observed in a study by Zhang and colleagues (2005) to validate the receptor’s effect on social behaviour and addiction. The A118G polymorphism of the μ-opioid receptor gene is hypothesized to be a loss-of-function allele that lowers mRNA expression and receptor protein translation rates (Zhang et al., 2005). It was observed from studies such as these that there is a greater physical pain susceptibility in childhood withdrawal, and even primates showing more pronounced and persistent separation anxiety (Tchalova & Eisenberger, 2015). Altogether, these research findings support the idea of shared sensitivities to physical and social pain and make a sound argument for the involvement of the endogenous opioid system in the regulation of social pain.
Opioid receptor antagonists, which are known to aggravate physical pain have also shown to increase distress vocalizations in isolated animals and slow the reduction in distress vocalizations typically seen when animals are reunited with their companions (Fisher, 2015). From these findings, it can also be argued that those prone to social anxiety, panic attacks and depression release less opioid, therefore taking longer to recover from negative social experiences. These individuals may also struggle to gain as much pleasure from social support as those who release more opioid in comparison (Fisher, 2015).
What is Hate?
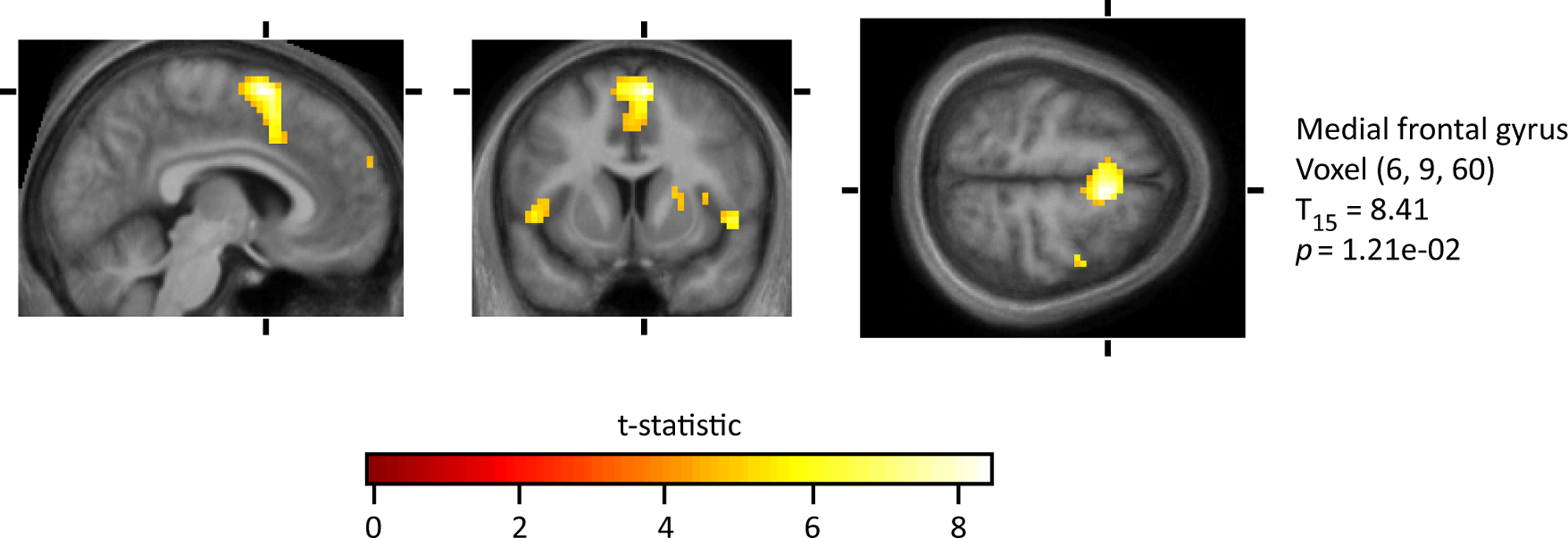
The opposite of love is hate. To hate, in its simplest form, is to have a strong emotional response of extreme dislike (Merriam-Webster, 2018). One can manifest a hatred for a variety of things, including an individual, a group, a society, culture, and race. Hate can also be demonstrated in a variety of ways as well- some believe that when one illustrates hatred, they are also demonstrating anger, while others believe that anger does not always have to be present to attain a feeling of hatred (Fitness & Fletcher, 1993). It has been found that when an individual experiences hatred, they also experience aggressive behaviour (although it should be noted that aggression and hate are two separate entities), heightened motor behaviour, and elevated neuronal activation in medial frontal gyrus, right putamen, premotor cortex, frontal pole, medial insula, right insula, right premotor cortex, and right front-medial gyrus (Zeki & Romaya, 2008). Thus, the simple definition of a ‘strong dislike’ is not a complete and incorporative definition of hatred, as it is a complex emotion with many different attributes and subsets.
Fundamentally, Robert Trivers (1971), an evolutionary biologist, proposed that hatred, in terms of the moral emotion directed to another individual, emerged as a way to regulate a system of reciprocal altruism. Altruism is behaviour that benefits another individual while lower your own inclusive fitness. Trivers explains that in a social setting, where friendships and relationships are able to be formed, altruism and reciprocity are key parts of the social system. If an individual breaks this system by taking advantage of this, hatred can emerge to isolate the individual and regulate how the individual will be treated in the future, by both the group and other individuals. Thus, a hatred to those who ‘cheat’ the system could have evolved via natural selection (Trivers, 1971). This term of punishing noncooperators of a group at the cost of one's self was later coined ‘altruistic punishment’ (Fehr & Gächter, 2002).
There are many other ideas of why individuals hate. One idea is that hate derives from fear of the unknown. The in-group out-group theory postulates that in times of fear or conflict our natural survival mechanism is to turn towards our ‘in group’, which are individuals who they identify with, love, or recognize. Thus, the ‘out-group’ are individuals who are collectively unrecognized and aggressed towards- thus leading to hate (Halevy, Bornstein, & Sagiv, 2008). Another idea is almost a mirror of the previous, where individuals hate others based on insecurities and fears of themselves. This is better known as ‘projection’, where individuals perceive their undesirable qualities onto others (Govorun, Fuegen, & Payne, 2006).
The Brain on Hate
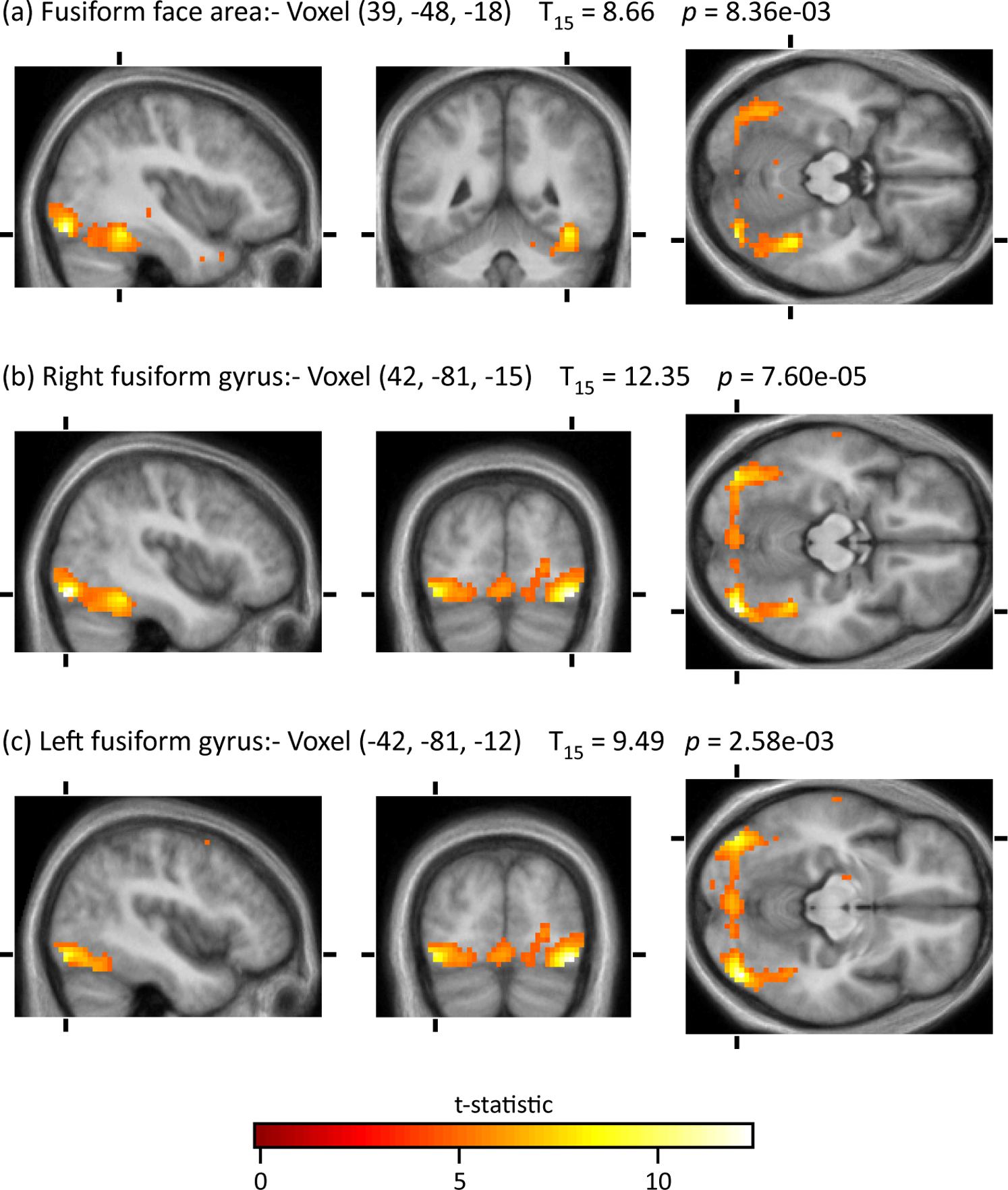
The reason why exactly we hate has been under discussion for decades, yet the exact cause has yet to be determined. However, in a groundbreaking study conducted by neurobiologists Zeki and Ramava (2008) they uncovered what they called, “The Hate Circuit.” In their study, 17 adults were scanned via fMRI while they first observed at the face of an acquaintance who they had neutral feelings towards, and then again when observing the face of someone they professed to hate. The areas activated in by the neutral faces were the same as when one would look at a blank face such as a mannequin, creating a baseline and control for the study (Zeki & Ramava, 2008) as seen in Figure 9.
However, when individuals were faced with the image of someone they hated, all 17 participants had the same four areas activated, in other words, a consistent “hate circuit” amongst the medial frontal gyrus, right putamen, premotor cortex and medial insula, as seen in Figure 10. Through further observations, these areas all we correlated linearly with the level of hatred participants felt, as well as activity for the initiation of aggressive behaviour, significantly different than anger or aggression itself (Zeki & Ramava, 2008). In particular, the premotor cortex and subcortex that was activated are related to the generation of aggressive behaviour, such as when combatting an individual or walking away from them, as well as general motor planning. Areas of the frontal cortex that were activated however were related to predicting the actions of others, which is key when in the decision-making processes when confronting a hated individual. In addition, it was observed the same two areas, the putamen, and the insula were activated when an individual is feeling either love or hate, indicating a biological connection between the two (Zeki & Ramava, 2008).
Potential Treatments
At its core, TC does not have an underlying treatment or preventive measure. When it is diagnosed, patients are usually treated as if they are having a heart attack, such as administration of aspirin, calcium channel blockers, cardiac stimulants, ACE inhibitors, and β-blockers. There is no long-term care reported (Komamura, Fukui, Iwasaku, Hirotani, & Masuyama, 2014). However, the following therapies were found to be effective in decreasing both physical and emotional pain associated with unpleasant social situations, such as heartbreak.
Opioid Drugs and Painkillers
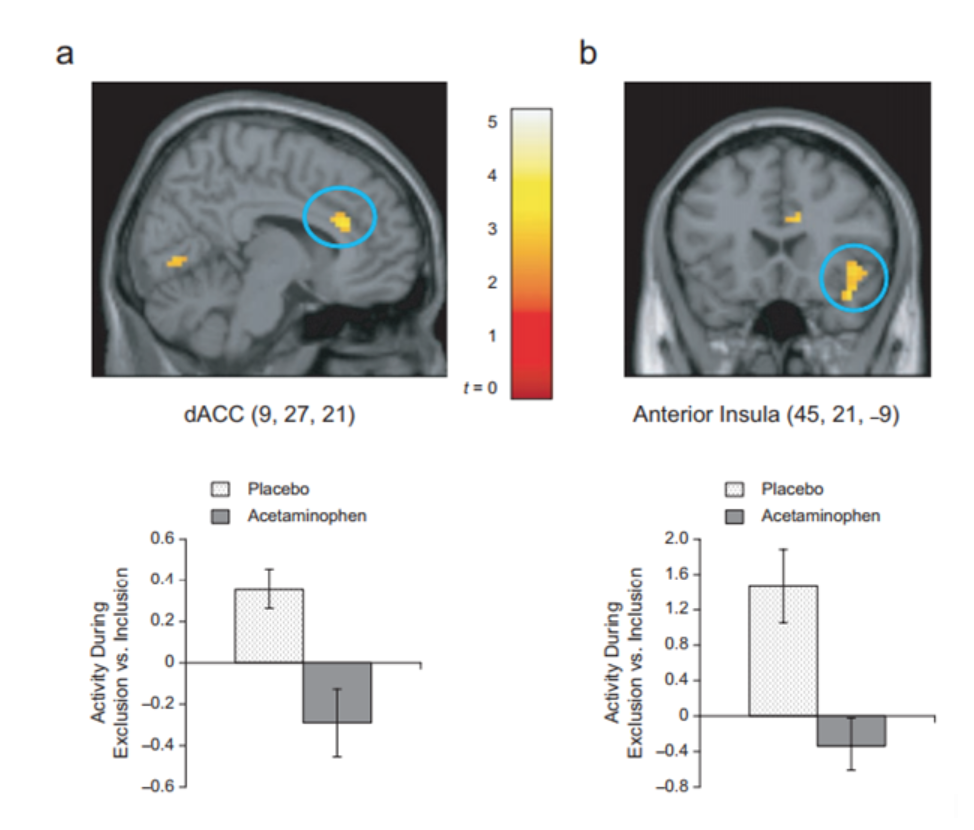
Opioid drugs are considered painkillers, but it has been found not to only diminish physical pain, but social pain as well. In several animal studies, it has been found that animals administered low-dose morphine, a type of opioid drug, lowered the frequency of social separation distress calls, while opioid receptor agonists increased the vocalizations from the isolated animals (Tchalova & Eisenberger, 2015). Painkillers are also effective at reducing both physical and social pain. To illustrate this, acetaminophen, also known generically as Tylenol, a common painkiller, was tested to see if it would reduce social pain as well as physical pain. Sixty-two healthy undergrad students were recruited for a study, in which they were instructed to take a 500-mg pill after waking up and before going to bed. 30 of the participants were given 1000mg Tylenol, while 32 were given a placebo. The participants were also instructed to use the Hurt Feelings Scale to report how much social pain they had endured that given day. The participants were to do this consecutively for 3 weeks. Following this, the patients were subjected to a functional MRI scale with efforts to observe differences in neuronal responses associated with social rejection in the brain. It was found that acetaminophen was associated with reduced social pain on a daily basis, and also reduced neuronal activation in the dorsal anterior cingulate cortex, and anterior insula- which are areas of the brain which are found to be associated with both physical and social pain (DeWall et al., 2010).
Figure 11. Both treatment (acetaminophen) and placebo group individuals were subject to fMRI full brain scans to observe neuronal activity. To highlight, the dorsal anterior cingulate cortex (dACC) (a) and right anterior insula (b) were both seen to have heightened activity in the acetaminophen group than the placebo group. (DeWall et al., 2010).
Placebo Treatments
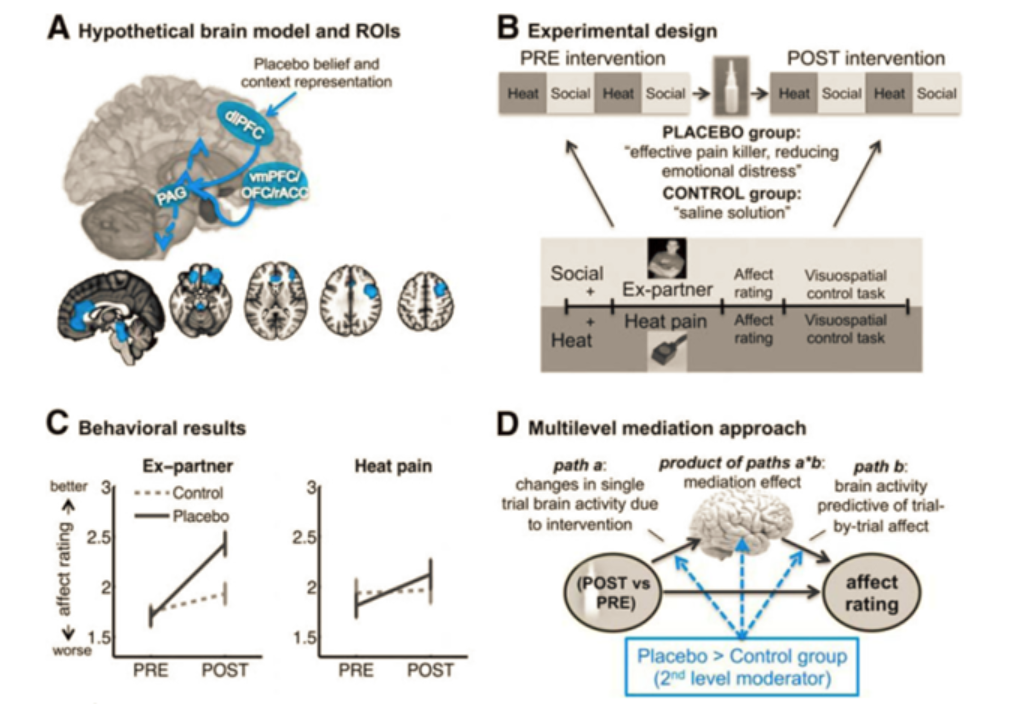
It is well documented that the placebos have significant effects on the perception of pain. In a study conducted in 2017 by Koban et al., researchers recruited 40 individuals who had recently experienced an “unwanted romantic breakup” and were asked to bring a picture of the past romantic partner (ex), as well as a same-gendered friend to the study. During the study, the participants were placed in a functional MRI and were asked to recall their previous relationship, while observing the picture of their ex. Then, they were shown images of their friend. During this time, the participants were also experiencing small bouts of physical pain induced by heat on their right arm. Thus, the fMRI would be able to observe the participants brain during emotional pain, as well as physical pain. Participants were asked to rate how they felt on a scale of 1 to 5, with 5 feeling very good. It was found that during this initial consult, it was found that similar areas of the brain were active during both physical and emotional pain. Following this experience, participants were given a saline nasal spray. Half of the individuals were told that the saline solution was a “powerful analgesic that is also effective in reducing emotional pain and negative affect” (placebo group), while the other half were told that the nasal spray was a saline solution to improve the contrast of the fMRI images. The above treatment was repeated with the displaying of the images, and heat stimulus. It was found that the placebo group experienced less physical and social pain, as well as an increase in the dorsolateral prefrontal cortex (which is an area of the brain that is involved in modulating emotions), periaqueductal gray (an area in the midbrain that regulates the levels of the release of opioids and excitatory neurotransmitters (Koban, Kross, Woo, Ruzic, & Wager, 2017). Social support- Social support helps alleviate the emotional and social distress, but also helps the physical pain. To highlight how social support helps with the physical pain, a study was conducted that observed females being subjected to thermally induced pain. Some individuals were either holding the hand, of their romantic partner or looking at a photo of their romantic partner. It was found that the women who were looking at the photos of their loved ones or holding their hand, experienced less pain compared to women who were alone. It is also found that social support during painful situations has led to decrease signaling in the dorsal anterior cingulate cortex and anterior insula, which are regions of the pain that perceive physical pain (Tchalova & Eisenberger, 2015).
Figure 12. Both treatment (acetaminophen) and placebo group individuals were subject to fMRI full brain scans to observe neuronal activity. To highlight, the dorsal anterior cingulate cortex (dACC) (a) and right anterior insula (b) were both seen to have heightened activity in the acetaminophen group than the placebo group. (DeWall et al., 2010).
Comfort Food
“Comfort food” is a term used to describe foods that satisfy an individual both physically and emotionally. It has been found in the past that individuals tend to eat these kinds of foods when they are emotionally unwell with the aim of feeling better emotionally. Highly palatable foods have been found to activate areas in one's brain associated with drug reward and pleasure (Avena, Gold, Kroll, & Gold, 2012). It has also been found comfort foods induce relationship-related cognitions, as well as lead to individuals feeling more belonged and secure (Troisi, Gabriel, Derrick, & Geisler, 2015). Lastly, it has also been found that palatable food can lead to the release of endogenous opioids (Adam & Epel, 2007).
Conclusion
In summary, love is a deep emotional experience and comprises of mental states ranging from affection to pleasures, influenced significantly by our desire or lust, attraction, and attachment towards someone. The more we experience these positive affections or pleasures, the more activated our brain’s reward circuit becomes. This acts as a positive feedback loop which is affected by multiple neurotransmitters and hormones that affect different components of love. There are many structures involved with the feeling of love including the caudate nucleus and the ventral tegmental area, also known as the reward circuit. These are also linked with the nucleus accumbens, the hippocampus, amygdala, and the prefrontal cortex. Within these structures, chemicals and hormones such as dopamine, norepinephrine, serotonin, vasopressin, oxytocin, testosterone and estrogen play an important role in the emotion of love. The mechanism of heartbreak is yet to be fully understood, but many journal articles propose sudden declines in dopamine and oxytocin, increased cortisol, as well as low opioid levels to be correlated to feelings of social separation distress, similarly observed in heartbreak. The “hate circuit” has also shown groundbreaking evidence on how certain areas of the brain, specifically the medial frontal gyrus, right putamen, premotor cortex and medial insula, are correlated to motor planning and predicting other’s behaviour when feeling hate towards someone. Overall love, heartbreak, and hate are complex human emotions that are equally as different, as they are similar.
Presentation
Click presentation_2_love_hate.pdf for a research seminar presentation on love and hate.
Copyright © Miguel Cardoso, Shara Chowdhury, Sabrina Musto, Harpreet Pabla, & Ojan Yarkhani
References
Adam, T. C., & Epel, E. S. (2007). Stress, eating and the reward system. Physiology and Behaviour, 91(4), 449–458. https://doi.org/10.1016/j.physbeh.2007.04.011
Avena, N. M., Gold, J. A., Kroll, C., & Gold, M. S. (2012). Further developments in the neurobiology of food and addiction: Update on the state of the science. https://doi.org/10.1016/j.nut.2011.11.002
Borreli, L. (2015, March 18). What Is Love? MRI Scan Reveals What Stages Of Romantic Love Your In Via Brain Map. Retrieved February 21, 2018, from http://www.medicaldaily.com/what-love-mri-scan-reveals-what-stages-romantic- love-youre-brain-map-326080
Burnett, D. (2016). The idiot brain: A neuroscientist explains what your brain is really up to.London: Guardian Faber.
Carter, C. S., & Porges, S. W. (2012). The biochemistry of love: an oxytocin hypothesis. EMBO reports,14(1), 12-16. doi:10.1038/embor.2012.191
Cingulate cortex. (2018). En.wikipedia.org. Retrieved 1 March 2018, from https://en.wikipedia.org/wiki/Cingulate_cortex
Deshmukh, A., Kumar, G., Pant, S., Rihal, C., Murugiah, K., & Mehta, J. L. (2012). Prevalence of Takotsubo cardiomyopathy in the United States. American heart journal, 164(1), 66-71.
DeWall, C. N., MacDonald, G., Webster, G. D., Masten, C. L., Baumeister, R. F., Powell, C., … Eisenberger, N. I. (2010). Acetaminophen Reduces Social Pain. Psychological Science, 21(7), 931–937. https://doi.org/10.1177/0956797610374741
Fehr, E., & Gächter, S. (2002). Altruistic punishment in humans. Nature, 415(6868), 137–140. https://doi.org/10.1038/415137a
Fisher, N. (2015). Rejection and physical pain are the same to your brain: Forbes. Retrieved from https://www.forbes.com/sites/nicolefisher/2015/12/25/rejection-and-physical-pain-are-the-same-to-your-brain/#58e12d144f87
Fisher, H., Aron, A., & Brown, L. L. (2005). Romantic love: An fMRI study of a neural mechanism for mate choice. The Journal of Comparative Neurology,493(1), 58-62. doi:10.1002/cne.20772
Fisher, M. (2018). The Science Behind Falling in Love. Psychology Today. Retrieved 26 February 2018, from https://www.psychologytoday.com/blog/loves-evolver/201302/the-science -behind-falling-in-love
Fisher, H. (2018). What is the Meaning of True Love l The Anatomy of Love. The Anatomy Of Love. Retrieved 26 February 2018,from https://theanatomyoflove.com/what-is-love/what-is-love/
Fitness, J., & Fletcher, G. J. O. (1993). Love, hate, anger, and jealousy in close relationships: A prototype and cognitive appraisal analysis. Journal of Personality and Social Psychology, 65(5), 942–958. https://doi.org/10.1037/0022-3514.65.5.942
Govorun, O., Fuegen, K., & Payne, B. K. (2006). Stereotypes Focus Defensive Projection. Personality and Social Psychology Bulletin, 32(6), 781–793. https://doi.org/10.1177/0146167205285556
Halevy, N., Bornstein, G., & Sagiv, L. (2008). “In-Group Love” and “Out-Group Hate” as Motives for Individual Participation in Intergroup Conflict. Psychological Science, 19(4), 405–411. https://doi.org/10.1111/j.1467-9280.2008.02100.x
Koban, L., Kross, E., Woo, C.-W., Ruzic, L., & Wager, T. D. (2017). Frontal-Brainstem Pathways Mediating Placebo Effects on Social Rejection. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 37(13), 3621–3631. https://doi.org/10.1523/JNEUROSCI.2658-16.2017
Komamura, K., Fukui, M., Iwasaku, T., Hirotani, S., & Masuyama, T. (2014). Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World Journal of Cardiology, 6(7), 602–9. https://doi.org/10.4330/wjc.v6.i7.602
Love. (2018). En.wikipedia.org. Retrieved 26 February 2018 from } https://en.wikipedia.org/wiki/Love
Loyola University Health System. (2014, February 6). What falling in love does to your heart and brain. ScienceDaily. Retrieved February 22, 2018 from www.sciencedaily.com/releases/2014/02/140206155244.htm
Merriam-Webster. (2018). Hate | Definition of Hate by Merriam-Webster. Retrieved February 22, 2018, from https://www.merriam-webster.com/thesaurus/hate
Moles, A., Kieffer, B. L., & D'amato, F. R. (2004). Deficit in attachment behavior in mice lacking the µ-opioid receptor gene. Science, 304(5679), 1983-1986.
Piomelli, D. (2001, October 01). Cannabinoid activity curtails cocaine craving. Retrieved February 21, 2018, from https://www.nature.com/articles/nm1001-1099
Takotsubo Cardiomyopathy - “Broken Heart Syndrome”. (2016). St. Vincent's Hospital Heart Health. Retrieved 1 March 2018, from https://svhhearthealth.com.au/Conditions/Takotsubo+Cardiomyopathy
Tchalova, K., & Eisenberger, N. (2015). How the Brain Feels the Hurt of Heartbreak: Examining the Neurobiological Overlap Between Social and Physical Pain. Elsevier , 3, 15–20. https://doi.org/10.1016/B978-0-12-397025-1.00144-5
Trivers, R. L. (1971). The Evolution of Reciprocal Altruism. The Quarterly Review of Biology, 46(1), 35–57. Retrieved from http://www.jstor.org/stable/2822435
Troisi, J. D., Gabriel, S., Derrick, J. L., & Geisler, A. (2015). Threatened belonging and preference for comfort food among the securely attached. Appetite, 90, 58–64. https://doi.org/10.1016/J.APPET.2015.02.029
Wu, K. (2018). Love, Actually: The science behind lust, attraction, and companionship - Science in the News. Science in the News. Retrieved 26 February 2018, from http://sitn.hms.harvard.edu/flash/2017/love-actually-science-behind-lust-attraction-companionship/
Zeki, S., & Romaya, J. P. (2008). Neural Correlates of Hate. PLoS ONE, 3(10), e3556. https://doi.org/10.1371/journal.pone.0003556
Zhang, Y., Wang, D., Johnson, A. D., Papp, A. C., & Sadée, W. (2005). Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. Journal of Biological Chemistry, 280(38), 32618-32624.