Table of Contents
Atrial Septal Defect
Atrial Septal Defect (ASD) is one of the most common type of congential heart disease (Geva et al., 2014). ASD contains many different types of arterial malformations causing the mixture of blood between the systemic and pulmonary circulation. Some individuals with ASD are asymptomatic because symptoms depends on the size and type of the defect (Briggs et al., 2012).
Embryology
Normal Heart Development
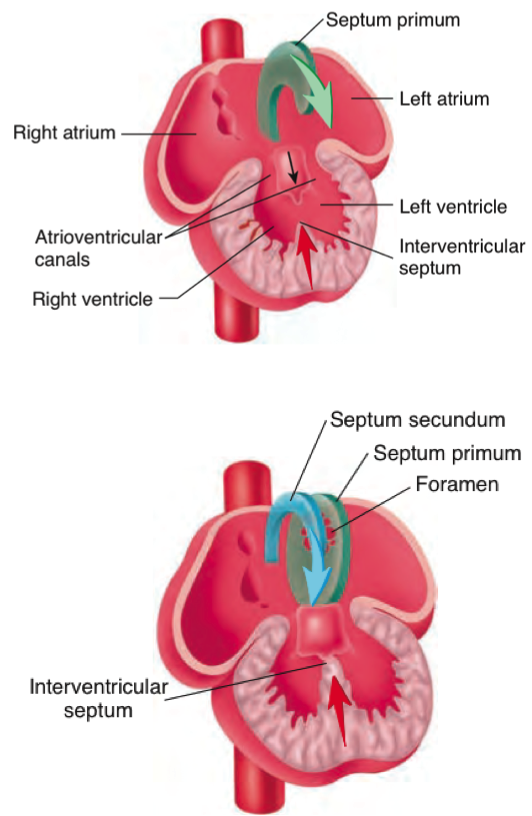
During human embryonic development the heart begins to grow 20 days after fertilization (Seeley & Tate, 2008). First, there are two parallel endocardia tube containing four regions: the sinus venosus, a single atrium, a single ventricle and the bulbus cordis. These tubes fuse together, elongate and bends into the loop. Parts of the loop extends forming the major chambers of the heart the atrium and ventricle, and starts to beat around 21-24 days. By 26 days the interatrial septum begins to form. Septum primum is the first atrial septum developed and as it develops the leading edge is called the mesenchymal cap (Geva et al., 2014). Around 35 days the a second septum septum secundum, grows right to the septum primum (Seeley & Tate, 2008). The atrial septum is nearly fully form at 60 days after fertilization and are crescent shaped structures (Geva et al., 2014). There is a interatrial septum opening called the foramen ovale allowing blood to flow from the right to left atrium because the fetus receives its oxygenated blood from the mother bypassing the lungs. The right and left atrium are fully define after birth when the lungs are functioning. The pulmonary gas exchange cause the left atrial pressure exceeds right atrial pressure. The difference of pressure results in the septum primum to push against the septum secundum and a close the foramen ovale (Geva et al., 2014).
Anatomy
Types of Atrial Septal Defect
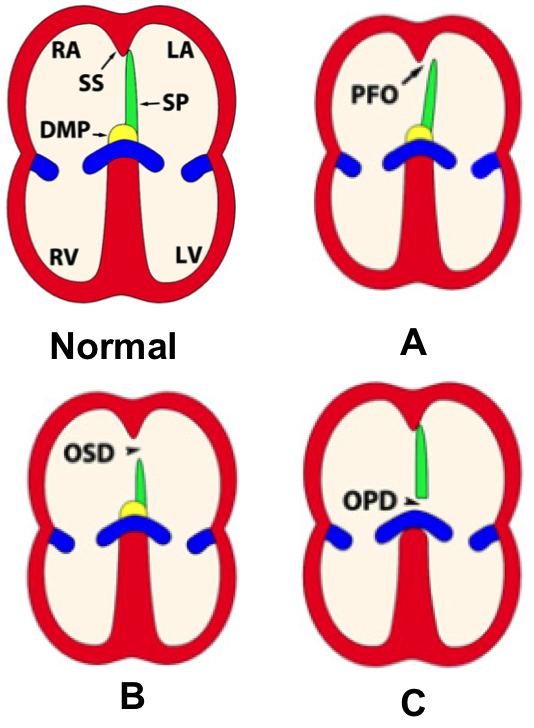
ASD arise due to complication in the development of the interatrial septum(Briggs et al., 2012). There are two main parts of the interatrial septum, septum primum and septum secundum. The septum primum is anchored by a muscular base which forms a floor of the fossa ovalis (Briggs et al., 2012). Also, the fossa ovalis is contributed by septum secundum. The three major kinds of ASD are patent foramen ovale, ostium secundum defect and ostium primum defect (Geva et al., 2014).
Patent Foramen Ovale
Patent foramen ovale (PFO) is a consequence of the septum primum and septum secundum failing to fuse (Briggs et al., 2012). Since the septums failed to fuse, there is a opening of the foramen ovale. Although PFO are presented in almost all newborns, it is estimated that PFO effects 20-34% of the adult population. Normally, PFO is asymptomatic because PFO is functionally close as long as the left atrial pressure exceeds that of the right atrium and the septums are sufficient in size (Briggs et al., 2012).
Ostium Secundum Defect
It is possible during development the septum primum size is insufficient and/or the septum secundum fails to fully develop causing ostium secundum defect (OSD) (Briggs et al., 2012). The opening of the interatrial septum of patients with OSD varies from several millimeters to three centimeters (Geva et al., 2014). OSD is the most common type of ASD.
Ostium Primum Defect
Ostium primum defect is less common and is an outcome of septation between the inferior margin of the septum primum and the atrial surface (Geva et al., 2014). Although incidence of OPD is lower than other types of ASD, OPD is more severe because it is usually associated with intraventricular septum openings, thus atrioventricular septal defects occurs (Briggs et al., 2012). Also, in atrioventricular septal defects the atrioventricular valves are abnormal causing problems in blood flows (Geva et al., 2014).
Pathophysiology
ASD is a hole in the septum between the right and left atria (Geva et al., 2014). This cause a left-to- right shunt where oxygenated blood from the left atrium is leaked into the right atrium. As a result the right chambers of the heart will experience a volume overload because there is more blood filling into the chambers. A volume overload correlates with pressure overload because more force is required to push the blood out into pulmonary circulation (Geva et al., 2014). As the heart over works itself, the right chambers of the heart (right atrium and right ventricle) enlarge. The size of the defect is associated with the extent of the symptoms such as defects smaller than 10 mm results in minimum shunt and minimum enlargement of the right side of the heart (Geva et al., 2014). As the right side of the heart enlarge, left ventricular diastolic filling decreases with left ventricular systolic dysfunction. Additionally the pulmonary to systemic flow ratio increase with decrease in systemic output (Geva et al., 2014).
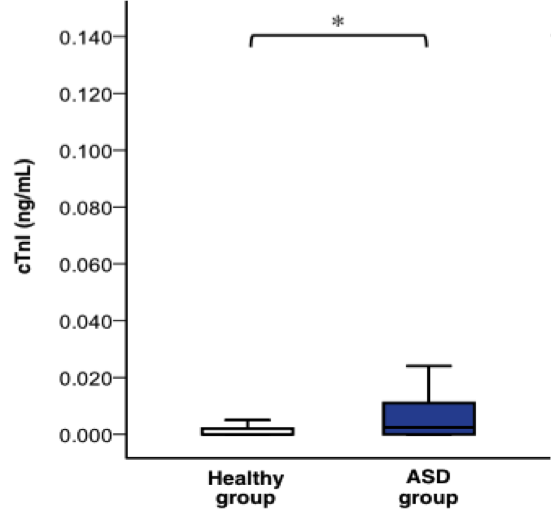
The pathophysiology effects of ASD is evident in an increased serum concentrations of Cardiac troponins (cTnI) (Geva et al., 2014). cTnI is a sensitive and specific biochemical marker which is release into the circulatory system as a response to cardiac injury (Sugimoto et al., 2010). Researchers measured the cTnI levels in healthy, ASD, and ventricular septum children ages two months to 16 years old (Sugimoto et al., 2010). The result shows ASD individuals have a significantly higher levels of these biomarkers compared to healthy individuals (P<0.05). According to Sugimoto et al., (2010) the high level of biomarkers is due to right side of the heart having a high volume overload.
Clinical Manifestations
Atrial septal defect is basically a hole in the wall between the two upper chambers in your heart. Atrial septal defect is present from birth and most often, small atrial septal defects close on their own during infancy or early childhood (Atrial Septal Defect, 2011).
Signs and Symptoms
There are not many concrete symptoms observed in individuals with atrial septal defect (Atrial Septal Defect, 2011). There are usually no associated signs or symptoms in babies born with atrial septal defect. Symptoms usually begin to appear in adults by the age 30 and some symptoms begin to appear decades later (Atrial Septal Defect, 2011). Some observable symptoms of atrial septal defects are:
• Fatigue
• Stroke
• Bluish Skin Color
• Heart Palpitations or skipped beats
• Heart Murmur, a whooshing sound that can be hear through a stethoscope
• Shortness of breath
• Swelling of abdomen, legs or feet
• Lung infection
• Feeling extremely exhausted after exercise
Causes
Until now, there has been no clear cause indicated for atrial septal defect (Atrial Septal Defect, 2011). The disease is present from birth and researchers state that due to errors in early heart development, atrial septal defect occurs. Also, researchers state that environmental or genetic factors might be the cause of the disease (Atrial Septal Defect, 2011).
The blood flow is disrupted in patients with atrial septal defect. Due to the whole in the wall between the two upper chambers in the heart, oxygenated blood flows from the left atrium into right atrium and mixes with deoxygenated blood. Here, the blood is pumped to the lungs even though it already has been supplied with oxygen already (Atrial Septal Defect, 2011). Due to the hole in the wall, volume of blood that flows into the right side of the heart can cause this side to enlarge and weaken over time. Because of the extra blood flow, pulmonary hypertension can occur due to increased blood pressure in the lungs (Atrial Septal Defect, 2011).
Risk Factors
Researchers have identified atrial septal defect to run in some families with history of this disease. Also, this disease is sometimes associated with genetic problems like Down syndrome (Atrial Septal Defect, 2011). Along with this, researchers have stated that during pregnancy if you get rubella infections or take drugs and alcohol, this will increase the chance of infant with a heart defect (Atrial Septal Defect, 2011). If the women has atrial septal defect, this can increase the risk of complications during pregnancy.
Complications
The complications of the atrial septal defect depend on how large the hole between the two upper chambers in your heart is (Atrial Septal Defect, 2011). Smaller defects usually close on their own during infancy or early childhood whereas, large defects can cause mild to life-threatening problems such as:
• Shortened life expectancy
• Higher chance of stroke
• Right side heart failure
• Heart Rhythm abnormalities
Some less common serious complications observed are such as:
• Pulmonary Hypertension: The blood pressure in the lung arteries increases due to increased blood flow to the lungs leading to pulmonary hypertension (Atrial Septal Defect, 2011).
• Eisenmenger syndrome: Sometimes pulmonary hypertension can lead to permanent lung damage and this state of condition is known as Eisenmenger syndrome. Eisenmenger syndrome is only observed in a small fraction of patients with heart defects and usually develops over a long period of time (Atrial Septal Defect, 2011).
Identification/Diagnosis
During a regular checkup, a doctor may suspect their patient to have an atrial septal defect if he or she hearts a heart murmur via the usage of a stethoscope. If a doctor is questioning this, they may seek out further testing to confirm the diagnosis. The following are the main tests that are requested:
Echocardiogram:
This is the most commonly used test to diagnose an atrial septal defect. In an echocardiogram, sound waves are used to help produce a video image of the heart. It is essentially like an ultrasound for the heart; where ultrasound waves are transmitted into the chest and the reflection of these waves give off various parts of the heart to be analyzed. What this allows is for the doctor to see both atriums and ventricles, measure their relative pumping strength and look for any heart defects or heart valve malfunctions (Sutton et al, 2014).
Chest X-Ray:
This test is usually issued to help cancel out any other potential conditions the patient might have that’s superimposed with the atrial septum defect. The chest x-ray can help a doctor see the condition of the heart and lungs (Sutton et al, 2014)
Electrocardiogram (ECG):
This test records the electrical activity of your heart and helps find any rhythmic heart problems.
Cardiac Catheterization:
This test is rarely used due to it being non-invasive towards the patient. It involves a thin, flexible tube called a catheter to be inserted into the blood vessel at the arm or groin and guided to the heart (Sutton et al, 2014). Through this, doctors can diagnose heart defects, test how well the heart is pumping and check heart valves.
Pulse Oximetry:
This is a painless test that measures oxygen levels in the tissues. The test involves a small clip on the fingertip that measures the oxygen levels (Sutton et al, 2014). It helps find whether or not oxygenated blood is mixing with deoxygenated blood. As a result, one can see what type of heart defect is present.
Management
Most patients with moderate to large ASDs will become symptomatic and require management of the defect by the age of 40. Intervention is necessary in the following cases (Conolly, 2014):
- High Qp (pulmonary flow)/Qs (systemic flow) ratio. Patients with a ratio of 1.5 or higher should receive intervention to close the defect.
- Right atrial or right ventricular enlargement with or without the following symptoms: Fatigue, Dyspnea, Heart Failure and Arrhythmia.
Methods of Intervention:
Surgical Closure. The procedure involves the following:
- Median Sternotomy which entails cracking open the sternum to gain access to the heart.
- Obtaining a patch the same size of the defect from the pericardium of the patient
- The patch is then stitched to the rim of the defect
- In cases when the patient is born lacking a pericardium, a bovine patch can be used.
(Conolly, 2014)
Percutaneous Closure
Half to two-thirds of the patients meet the criteria for the procedure. The eligibility criteria include:
- The patient must have Secundum ASD
- And the size of the defect must be less than 30 mm.
(Conolly, 2014)
One of the devices used is the Amplatzer Occlusion Device which is made up of a right and left nitinol wire mesh discs connected together which are delivered to the heart through catheterization.
Surgery, though rendered statistically more effective than percutaneous closure, is associated with longer hospital stays and post-operative complications (Sutton, 2014).
Conclusion
ASD is an aperture in the septum between the right and left atria. The true cause for ASD is still a mystery but when symptomatic can cause serious complications such as heart murmur, stroke, and bluish skin color. Echocardiogram, Chest X-Ray and Electrocardiogram are just some examples of diagnosis testing. Surgery, though rendered more effective through statistics, is associated with longer hospital stays and postoperative complications. Research is needed to improve efficacy of non-invasive devices.
References
Atrial Septal Defect. (2011). Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/atrial-septal-defect/in-depth/CON-20027034
Briggs, L. E., Kakarla, J., & Wessels, A. (2012). The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation, 84(1), 117-130.
Connolly, HM. Management of atrial septal defects in adults. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on November 26, 2014.)
Geva, T., Martins, J. D., & Wald, R. M. (2014). Atrial septal defects. The Lancet,383(9932), 1921-1932.
Seeley, R., & Tate, P. (2008). Anatomy & physiology (8th ed.). Dubuque, IA: McGraw-Hill.
Sugimoto, M., Ota, K., Kajihama, A., Nakau, K., Manabe, H., & Kajino, H. (2010). Volume overload and pressure overload due to left-to-right shunt-induced myocardial injury.-Evaluation using a highly sensitive cardiac Troponin-I assay in children with congenital heart disease-. Circulation journal: official journal of the Japanese Circulation Society, 75(9), 2213-2219.
Sutton, J., et al. (2014). Identification and assessment of atrial septal defects in adults. UpToDate. (Accessed on November 22, 2014.)
Sutton, MG. Devices for percutaneous closure of a secundum atrial septal defect. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on November 26, 2014.)