Table of Contents
Parkinson's Disease
Parkinson’s disease is a neurodegenerative brain disorder that results in individuals to experience loss of control of their muscles (Parkinson’s Society Canada, 2014). This disease occurs extremely slowly and progressively; meaning that as time passes, individuals can expect to experience worsening and more severe symptoms. Along with this, there is not an acute phase to this disease. It is chronic and is something that will remain with someone who has Parkinson’s disease for the rest of his or her lifetime. Luckily, the primary effects of Parkinson’s disease are not fatal and many of those who pass from it is because of secondary symptoms like pneumonia or choking (Parkinson’s Society Canada, 2014). However, many can expect to live healthy lives with this disease. Currently, there is no known cure for Parkinson’s disease. Thus, research is continuously being conducted towards the disease, specifically looking for a cure or treatment methods to combat against the disease.
Parkinson’s disease is the second most common age-related neurodegenerative disease following Alzheimer’s disease. It affects seven million people globally with majority of those being the elderly of around the age of 60 (Parkinson’s Society Canada, 2014). Around 5-10% of Parkinson’s cases take place with individuals of ages 20 to 50 (Parkinson’s Society Canada, 2014). When looking at the difference between genders, men are found to be more likely to develop Parkinson’s compared to women.
Cause
Parkinson’s disease occurs due to the damage, loss and degeneration of a small part of the brain called the substantia nigra (National Parkinson Foundation, 2013). Within this area, there is a high concentration of dopamine-producing cells. The dopamine molecules produced allow brain cells involved in movement control to communicate with each other. More specifically, it allows the relaying of messages between the substantia nigra and cerebral cortex/cerebellum (Body and Health, 2012). When these dopamine-producing cells are destroyed, dopamine levels in the brain decrease. As a result, Parkinson’s disease arises with up to 80% of the cells in the substantia nigra being destroyed.
Signs and Symptoms
Individuals who have Parkinson’s disease display four key set of signs and symptoms:
- Tremor – this is one of the most recognized symptoms of Parkinson’s disease. This involves the involuntary shaking movement of the limbs, head and entire body. Usually, the tremor begins in one location of the body and progressively spreads to other areas (Parkinson’s Disease Foundation, 2014). Along with this, many experience a resting tremor, which involves one to be remaining in a stationary position but still shaking. Around 70% of people with Parkinson’s experience the symptom of tremor (Parkinson’s Disease Foundation, 2014).
- Bradykinesia – this is one of the more classic symptoms of Parkinson’s disease. It involves the slowness of movement or the complete loss of movement (Parkinson’s Disease Foundation, 2014). This can result in individuals with Parkinson’s to experience stooped posture, a shuffled walk, quit speech or paralysis.
- Ridgity – this is found in most people with Parkinson’s. It is the visible stiffness of the limbs and spinal column (Parkinson’s Disease Foundation, 2014). Due to the tenseness and constant contraction of muscles, individuals are unable to swing their arms when walking and experience a decrease in their range of motion
- Posture Instability – this is impaired balance and coordination, which involves individuals to have a stooped posture with, drooped shoulders and bowed down head (Parkinson’s Disease Foundation, 2014). Along with this, many develop a forward or backward lean, which can result in many falls and injuries.
Non-Motor Symptoms:
- Loss of Smell
- Choking and swallowing issues
- REMs Sleep - physically moving while sleeping, shouting, etc.
- Orthostatic Hypotension - decrease in blood pressure when moving upright
- Fatigue
- Depression
- Fear/Anxiety
Clinical Diagnosis
There currently are not any biological markers that can be tested in order to detect if an individual has Parkinson’s disease. Therefore, in order to diagnose an individual with Parkinson’s disease, a doctor would have to notice one of the four main motor symptoms, along with seeing some overlap with the non-motor symptoms.
Pathogenesis
Parkinson disease is influenced by genetic factors by causing protein aggregation, mitochondrial or lysosomal dysfunction, and effects in cellular signaling pathways. Currently there are 16 loci (PARK1 to PARK16) and 11 genes associated with Parkinsonism.(Corti et al., 2011).Some of the identified genes are α-synuclein (SNCA), parkin, PTEN-induced kinase 1 (PINK1), DJ-1, leucine-rich repeat kinase 2 (LRRK2) and ATPase type 13A2 (ATP13A2). The mode of inheritance is either autosomal dominant or recessive (Shin et al., 2009).
LRRK2
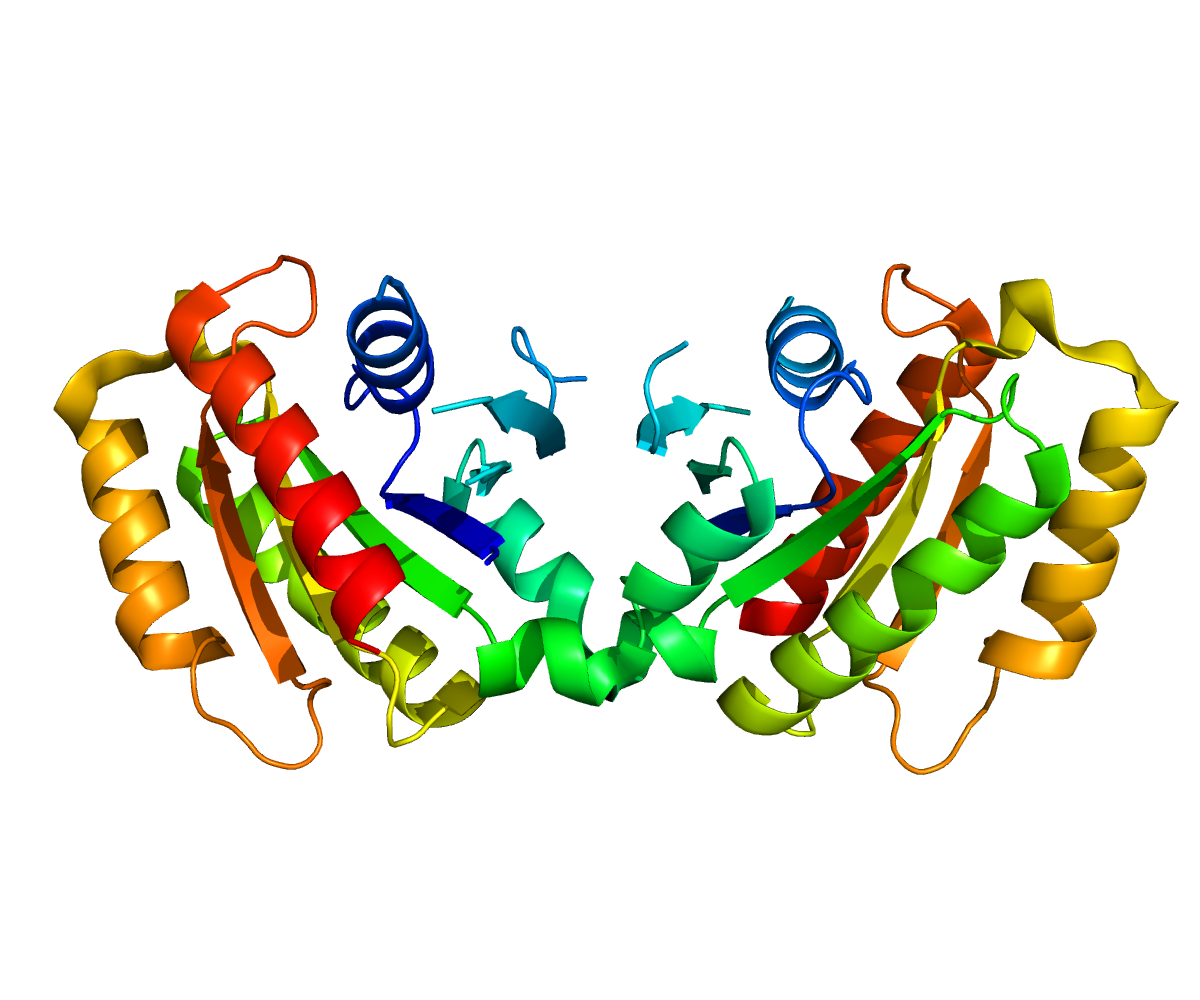
Structure
Leucine-rich repeat kinase 2 (LRRK2) gene has been of interest since its locus (PARK8), was mapped from a large Japanese family with autosomal dominant Parkinson’s disease (Funayama et al., 2005). On chromosome 12, this gene spans a genomic distance of 144kb with 51 exons (Corti et al., 2011). LRRK2 gene encodes for a large multidomain protein, with 2,527 amino acids (Raquel Esteves et al., 2014). This protein is also called “Dardarin” to reflect the discovery of its first mutation in a Basque family with tremor (Paisan-Ruiz et al., 2004). From the N- to C- Termini, the conserved domains within this protein are armadillo repeat folds (ARM), ankyrin repeat (ANK), leucine-rich repeat (LRR) domain, a Ras of complex protein (ROC), COOH terminal of Roc domain (COR), kinase domain (TKL) and WD40 domain (Mata et al., 2006). Since LRRK2 possess structural characteristics such as a ROC-COR region, LRR, and a WD40 domain, the LRRK2 is considered a member of the ROCO protein family (Esteves et al., 2014).
Function
The multidomain protein structure of LRRK2 suggests it may have the ability to modify multiple signaling pathways. Similar to a homologous protein LRRK1, LRRK2 encodes for two enzymes, kinase and GTPase within the same molecule (Greggio & Singleton, 2007). At the Thr558 site of LRRK2, the optimal substrate to induce kinase activity is moesin/LRRKtide (Jaleel et al., 2007). The phosphorylation of this substrate may influence the cytoskeleton anchoring to the plasma membrane (Mangeat et al., 1999). LRRK2, may also promote translation by phosphorylating the 4E-BP-eIIF4E complex (Imai et al., 2008). Additionally, LRRK2 has the capabilities to autophosphorylated (Gloeckner et al., 2009). There is a possibility of some degree of kinase-GTPase regulation since the autophosphorylation sites are localize within the kinase and ROC domain (Greggio et al., 2009).
LRRK2 is located in cytoplasm and is linked with many intracellular membrane and vesicular structures. Although several studies explored LRRK2 interactions with other components of the cell, the exact physiological functions of this protein are not well characterized (Esteves et al., 2014).
Normal Function of LRRK2: Nl2D5HUUsHc?.swf
LRRK2 Mutation: d0S7dPfxe_U?.swf
Clinical significance:
Only 10% of autosomal dominant familial Parkinson’s disease patients, 3.6% of sporadic Parkinson’s disease patients and 1.8% of healthy control patients contains some form of LRRK2 mutations (Corti et al., 2011). It is possible to have healthy carriers of this mutant gene because this gene’s penetrance is incomplete. This genetic mutation has a late onset for the reason that symptoms generally developed after 60 to 70 years (Esteves et al., 2014). Individuals with these mutations may experience typical Parkinson’s disease symptoms such as tremor, postural instability, rigidity and bradykinesia (Haugarvoll and Wszolek, 2009). Through the autopsies of individuals with LRRK2 mutation, it is noted presences of neurodegeneration in the substantia nigra and an absences Lewy bodies.
Due to many experimental studies it is believed that the majority of LRRK2 mutations are missense mutation with gain-of-function pathogenic mechanism (Cookson, 2010). There are at least seven known LRRK2 mutations, which relates to kinase or GTPase activity. These mutations influence relations between LRRK2 protein and dopamine homeostasis, vesicle trafficking, α-synuclein protein, protein quality control mechanisms, mitochondria, or cytoskeleton (Esteves et al., 2014).
The most common missense mutation is G2019S. This particular LRRK2 mutation is linked with autosomal dominant and apparent sporadic PD patients (Esteves et al., 2014). This mutation is located at the kinase domain and occurs when a glycine residue is exchanged for serine residue. G2019S influences the LRRK2 protein to increase its kinase activity because this structural change decreases the flexibility of this protein into a lock active conformation (Greggio & Cookson, 2009). The increase in kinase activity introduces neuronal toxicity to the cell and disrupts the ubiquitin-proteasome system (Shin et al., 2009). In transgenic mice it has been shown that increased expressions of G2019S LRRK2 induced age dependent loss nigrostriatal dopamine neurons, abnormal mitochondria and reduced neurite complexity (Ramonet et al., 2011).
I2020T and I2012T mutations are located in the kinase domain. However, its’ sole influences in LRRK2 protein function are uncertain at this time because of the inconsistency in experimental results (Esteves et al., 2014). Yet, it appears that 12020T and G2019S mutation increased tau-phosphorylation that promotes cell aggregation (Kawakami et al., 2012).
Familial Parkinson’s disease is associated with a LRRK2 mutation of the R1441(C/G/H) position in ROC domain and/or a Y1699C mutation in the COR domain. It is perceived that mutation in the ROC-COR domain lowers GTPase activity by prolonging the active GTP-bound state (Corti et al., 2011). Shown in cortical neurons of mice, the expression of R1441C altered calcium homeostasis and induced mitochondrial degradation (Cherra et al., 2013). Additionally, it appears that an R1441G mutation interferes with the interaction between LRRK2 protein and microtubules and thus, affects the satiability and acylation of tubulin (Law et al., 2013).
A N437H mutation on the GTPase domain of the LRRK2 protein is associated with Parkinson’s disease. Similar to mutation on the ROC-COR domains, N437H mutation decreases GTPase activity. However, GTP binding to the LRRK2 protein increases not the prolonging of the active GTP-bound state (Rudenko et al., 2012).
A substitution of arginine for glycine in the C-terminal WD40 domain of the LRRK2 protein decreases kinase activity (Rudenko et al., 2012). Like the Y1699C mutation, the R1628P mutation is located in the COR domain. Asian individuals with either of this mutation, have a two to threefold increase risk of developing idiopathic Parkinson’s disease (Corti et al., 2011).
Current Treatments
Over the past years, extensive research has been done to find a permanent cure for Parkinson’s Disease. The affects of Parkinson varies from person to person as it is a complex disease. Some treatments that have been proven to be effective against this disease are Deep Brain Stimulation and Gene Therapy.
Deep Brain Stimulation
The National Institute of Neurological Disorders and Stroke are continuously researching on Deep Brain Stimulation and developing more ways to improve it. Deep brain Stimulation is basically a surgical procedure used to treat several disabling neurological symptoms of Parkinson’s Disease such as rigidity, tremor and dystonia. Studies have shown the Deep brain stimulation proves to be most successful after taking regular medication for Parkinson’s Disease. Deep Brain Stimulation involves the use of an implantable pulse generator (IPG), which is similar to a heart pacemaker and about the size of a stopwatch (Deep Brain Stimulation, 2014). The implantable pulse generator works by delivering electrical stimulation to parts of the brain that control movement. These electrical simulations then end up blocking the abnormal nerve signals that cause Parkinson's Disease symptoms. The implantable pulse generator is generally implanted in the lower chest or near the collarbone(Deep Brain Stimulation, 2014). Other than the implantable pulse generator, components such as lead (electrode) and the extension are also incorporated into the surgical procedure. The lead is implanted in the brain through a small opening in the skull which touches the specific area in the brain and the extension is passed under the skin of the neck that connects the implantable pulse generator and lead (Deep Brain Stimulation, 2014). Before the procedure can be carried out, a neurosurgeon locates the target position in the brain. A neurosurgeon can accomplish by either using computed tomography (CT) or magnetic resonance imaging (MRI)(Deep Brain Stimulation, 2014). Deep Brain Stimulation is not a permanent cure to Parkinson's Disease but it alleviates several symptoms of the disease.
A research team led by Dr. Craig van Horne at University of Kentucky in America, are trying to combine the Deep Brain Stimulation procedure with grafting of a patient's peripheral nerve tissue into the brain (University of Kentucky, 2013). Peripheral nerves have the ability to regenerate if they are damaged by releasing neurotrophic factors which are protein-like molecules. These protein-like molecules support the growth of neurons (University of Kentucky, 2013).If nerves in the brain are damaged, they are not able to regenerate but peripheral nerves are nerves outside the spinal cord and brain. The research team at University of Kentucky anticipates that the regenerative ability of the peripheral nerves will allow the brain to heal itself (University of Kentucky, 2013). This procedure is carried out by obtaining the peripheral nerve tissue from just above the patient's ankle. This procedure does not require a separate surgery as the nerve tissue is implanted during the Deep Brain Stimulation surgery. This method is currently being looked into further as it could potentially yield promising results.
Gene Therapy
Another more current treatment which promises to yield promising results for Parkinson's Disease is Gene Therapy. This approach was formed by Professor Nicholas Mazarakis at Imperial College London and the treatment is called ProSavin. This method basically uses a harmless modified virus, Lentivirus to deliver three dopamine-making genes to a part of the brain that controls movement, the Striatum (Wong, 2014). Dopamine is a chemical that is used by nerve cells in the brain to control muscle movement and Parkinson's causes dopamine making brain cells to slowly die. Dopamine production is boosted by current treatments but only temporarily as the cells then begin to degenerate until the treatment no longer becomes effective (Wong, 2014). This treatment by Professor Nicholas aims to find a solution to this by inducing dopamine to be permanently produced in different sets of cells thereby providing a long-term solution (Wong, 2014). This gene therapy treatment was first tested in rats and the end results showed the treatment to be a success and only after this, human trials were carried out. Until now, the treatment has not shown any serious side effects. PET scans have shown higher dopamine levels in the affected areas of the brain six months after the treatment (see Figure below). The patients have been reported to be feeling better than before. However, the treatment is currently very expensive. The researches expect the costs to go down as more health care institutes slowly support their research.
Gene Therapy for Parkinson's Disease: 3gVn3BKXAWo?.swf
Treatments Under Trial
Cell Therapy
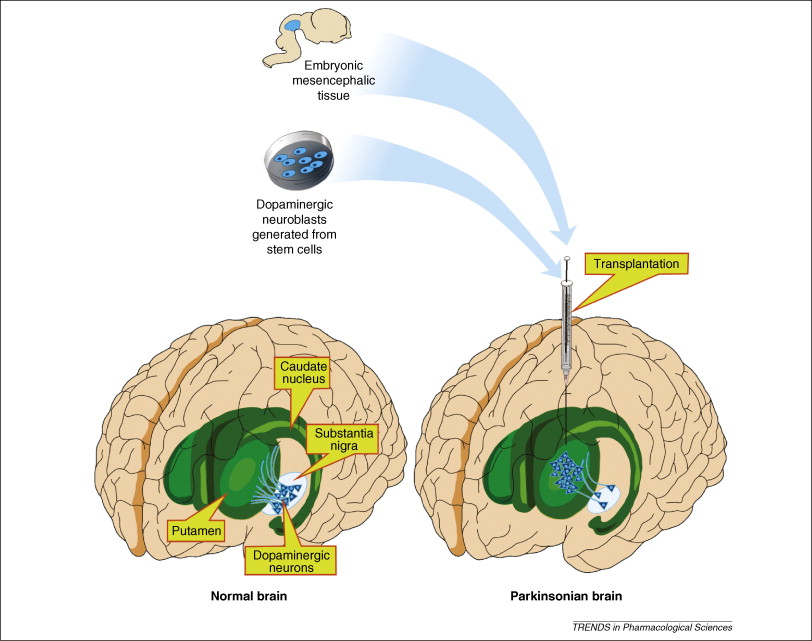
Cell therapy via intrastriatal transplantation of dopamine-rich human embryonic mesencephalic tissue is one of the potential treatment regimens for Parkinson’s Disease (PD) that remains under trial. The use of this therapy is meant to replace pharmacological treatments such as levodopa, which is associated with multiple side effects and is only effective in the initial stages of the disease (Alizadeh et al, 2013).
An open-label trial conducted by Brunden et al in 2000 showed promising results. Five patients from Sweden, London and Munich were recruited for the study. Each patient received tissue from 7-9 aborted embryos and the neuroblasts were transplanted bilaterally into the caudate and putamen nuclei using MRI and CT guided stereotaxic surgery.
Four of the five patients experienced significant post-operative improvements in swallowing, speech, gait, posture and postural stability. A few of the results are summarized below:
- Medication Dose: All patients reduced L-dopa by 54% average
- Clinical Status: Patient 15: no change post-operatively. Patients 12, 13, 14 and 16: Marked decrease in Unified Parkinson’s Disease Rating Scale (UPDRS) motor examination scale by 48%
- Graft Viability (measured by Ki, flourodopa influx rate constant): Putamen: 66% Left and 55% Right. Caudate: 24 % increase.
It has been proposed that the differences in graft viability between left and right sides of the putamen might have been due to a post-surgical inflammatory response that hinders the survival of one of the grafts (Brunden et al, 2000).
One of the obstacles facing this procedure is the rate of survival of the grafted neurons in the recipient. Only a small portion of them survive due to graft rejection and immune responses initiated by the host in reaction to the foreign antigens. Studies show the rate of survival of neurons after grafting to be as low as 3-20%. A significant finding of this study, however, involves the use of the chemical, Lazaroid. Lazaroid reduces the effects of oxidative damage which helps to increase the rate of dopaminergic neuron survival. That was demonstrated through the use of 42% and 50% less tissue than previous trials in the putamen and caudate respectively (Brunden et el, 2000).
An additional main challenge to this technique is the large amounts of human embryonic mesencephalic tissue that need to be grafted in patients in order for any significant therapeutic effect to occur. It has been shown that the tissue from at least 3-4 human embryos is needed to elicit change in one PD patient. Finally, the use of cell therapy to achieve dopaminergic re-innervation in the nigrostriatal circuit does not provide any relief to non-dopaminergic symptoms (Brunden et al, 2000).
Conclusion
Parkinson's Disease is a complex disease as it is a disorder of the nervous system that affects your movement. Over time, the symptoms of the disease worsen starting from a tremor in just one hand to slowed body movement. Currently there is no permanent cure for Parkinson's Disease, medications and several effective treatments such as Deep Brain Stimulation and Gene Therapy can be done to alleviate several symptoms of the disease.
References
Alizadeh, R., Mehrabi, S., & Hadjighassem, M. (2013). Cell Therapy in Parkinson’s Disease. Archives of Neuroscience, 1(2), 43-50.
Body and Health. (2012). Parkinson’s Disease. Retrieved from http://bodyandhealth.canada.com/channel_condition_info_details.asp?channel_id=2046&disease_id=102&relation_id=33685#Causes
Brundin, P., Pogarell, O., Hagell, P., Piccini, P., Widner, H., Schrag, A., … & Lindvall, O. (2000). Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain,123(7), 1380-1390.
Cherra III, S.J., Steer, E., Gusdon, A.M., Kiselyov, K., Chu, C.T., 2013. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am. J. Pathol. 182, 474–484.
Cookson, M. R. (2010). The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nature Reviews Neuroscience, 11(12), 791-797.
Corti, O., Lesage, S., & Brice, A. (2011). What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiological reviews, 91(4), 1161-1218.
Deep Brain Stimulation. (2014). National Institute of Neurological Disorders and Stroke. NINDS. Retrieved from http://www.ninds.nih.gov/disorders/deep_brain_stimulation/deep_brain_stimulation.htm
Esteves, A.R, Swerdlow, R. H., & Cardoso, S. M. (2014). LRRK2, a puzzling protein: Insights into Parkinson’s disease pathogenesis. Experimental Neurology.
Funayama, M., Hasegawa, K., Ohta, E., Kawashima, N., Komiyama, M., Kowa, H., … & Obata, F. (2005). An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Annals of neurology, 57(6), 918-921.
Gloeckner, C.J., Schumacher, A., Boldt, K. & Ueffing, M., 2009. The Parkinson disease- associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. J. Neurochem. 109, 959–968.
Greggio, E. & Singleton, A., 2007. Kinase signaling pathways as potential targets in the treatment of Parkinson's disease. Expert Rev. Proteomics, 4, 783–792.
Haugarvoll, K. & Wszolek, Z.K., 2009. Clinical features of LRRK2 parkinsonism. Parkinsonism Relat. Disord. 15 (Suppl. 3), S205–S208.
Imai, Y., Gehrke, S., Wang, H.Q., Takahashi, R., Hasegawa, K., Oota, E., Lu, B., 2008. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J, 27, 2432–2443.
Jaleel, M., Nichols, R., Deak, M., Campbell, D., Gillardon, F., Knebel, A., & Alessi, D. (2007). LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem. J, 405, 307-317.
Kawakami, F., Yabata, T., Ohta, E., Maekawa, T., Shimada, N., Suzuki, M., … & Obata, F. (2012). LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS One, 7(1), e30834.
Law, B.M., Spain, V.A., Leinster, V.H., Chia, R., Beilina, A., Cho, H.J., Taymans, J.M., Urban, M. K., Sancho, R.M., Blanca Ramirez, M., Biskup, S., Baekelandt, V., Cai, H., Cookson, M.R., Berwick, D.C. & Harvey, K., 2013. A direct interaction between Leucine-rich Repeat Ki- nase 2 and specific beta-tubulin isoforms regulates tubulin acetylation. J. Biol. Chem. 289 (2), 895–908.
Lindvall, O., & Kokaia, Z. (2009). Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends in pharmacological sciences, 30(5), 260-267.
Mangeat, P., Roy, C. & Martin, M., 1999. ERM proteins in cell adhesion and membrane dynamics: authors' correction. Trends Cell Biol, 9, 289.
Mata, I. F., Wedemeyer, W. J., Farrer, M. J., Taylor, J. P., & Gallo, K. A. (2006). LRRK2 in Parkinson's disease: protein domains and functional insights. Trends in neurosciences, 29(5), 286-293.
National Parkinson Foundation. (2013). What is Parkinson’s Disease. Retrieved from http://www.parkinson.org/parkinson-s-disease/pd-101/what-is-parkinson-s-disease
Parkinson Society Canada. (2014). What is Parkinson’s? Retrieved from http://www.parkinson.ca/site/c.kgLNIWODKpF/b.5184077/k.CDD1/What_is_Parkinsons.htm
Parkinson’s Disease Foundation. (2014). Primary Motor Symptoms. Retrieved from http://www.pdf.org/symptoms_primary
Paisán-Ruı́z, C., Jain, S., Evans, E. W., Gilks, W. P., Simón, J., van der Brug, M., … & Singleton, A. B. (2004). Cloning of the Gene Containing Mutations that Cause PARK8 -Linked Parkinson's Disease. Neuron, 44(4), 595-600.
Ramonet, D., Daher, J. P. L., Lin, B. M., Stafa, K., Kim, J., Banerjee, R., … & Moore, D. J. (2011). Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One, 6(4), e18568.
Rudenko, I. N., Chia, R., & Cookson, M. R. (2012). Is inhibition of kinase activity the only therapeutic strategy for LRRK2-associated Parkinson's disease?. BMC medicine, 10(1), 20.
Shin, J. H., Dawson, V. L., & Dawson, T. M. (2009). SnapShot: pathogenesis of Parkinson's disease. Cell, 139(2), 440-e1.
University of Kentucky. (2013). Trial of promising new treatment strategy for Parkinson's disease. ScienceDaily. Retrieved from www.sciencedaily.com/releases/2013/11/131119131045.htm
Wong, S. (2014). Health Care. Imperial College London. Retrieved from http://www3.imperial.ac.uk/newsandeventspggrp/imperialcollege/newssummary/news_9-1-2014-16-19-43