Table of Contents
Alzheimer's Disease
Presentation File: alzheimer.pdf
Introduction
Alzheimer’s disease (AD) is an irreversible brain disorder that is characterized by a progressive decline in cognitive function, which typically begins with deterioration in memory (Alzheimer’s Disease, 2016). Alzheimer’s is the most common neurodegenerative disorder, and is the sixth leading cause of death in the United States (Alzheimer’s Disease, 2016). The greatest known risk factor is increasing age, however Alzheimer’s is not a normal part of aging as one can also develop early-onset Alzheimer’s in their 40s or 50s of age (Alzheimer’s Disease, 2016). Alzheimer’s disease is a degeneration of neurons in the brain, starting in the temporal lobe and spreads to parietal, and frontal lobe(Alzheimer’s Disease, 2016)). The overall mass of the brain is reduced as a result of cell death, and the degeneration of neurons (Alzheimer’s Disease, 2016)). Alzheimer’s disease is progressive, and has three stages : early, mild to moderate, and severe (Alzheimer’s Disease, 2016)). In the earlier stages, memory loss is mild compared to the late stages, where the ability to carry a conversation and respond to the environment is completely impaired, resulting in complete dependence on others for care (Alzheimer’s Disease, 2016). There is no cure for Alzheimer’s that stops the progression completely, however treatments are available for the symptoms and can temporarily slow the worsening of the symptoms, and improve quality of life (Alzheimer’s Disease, 2016).
History
The history of Alzheimer’s disease dates back to 1906 (Hippius & Neundörfer, 2003). Alois Alzheimer’s was a German psychiatrist and neuropathologist. In 1901, Alzheimer’s observed a female patient, Auguste Deter who was 51-year-old, and had unpredictable behavioural symptoms including a loss of short-term memory (Hippius & Neundörfer, 2003). Deter became Alzheimer’s obsession, and he requested that she remained at the Frankfurt asylum where he was able to receive her records and brain upon her death in 1906 (Hippius & Neundörfer, 2003). Alzheimer’s used staining techniques to examine the brain, and identified amyloid plaques and neurofibrillary tangles present (Hippius & Neundörfer, 2003). These brain abnormalities identified were concluded to be indicators of Alzheimer’s disease as a result of Dr. Alois Alzheimer’s obsession (Hippius & Neundörfer, 2003). The diagnosis of Alzheimer’s disease was initially specific for individuals between the ages of 45 and 65 who developed symptoms of dementia. The terminology changed after 1977, which had led to the diagnosis of Alzheimer’s disease independent of age (Hippius & Neundörfer, 2003). Senile dementia of the Alzheimer type (SDAT) was a term used to describe the condition for individuals over the age of 65, and classical Alzheimer’s disease used to describe those that were younger (Hippius & Neundörfer, 2003). Eventually, the term Alzheimer’s disease was adopted to describe individuals of all ages with a characteristic common symptom pattern, disease course, and neuropathology (Hippius & Neundörfer, 2003).
Epidemiology
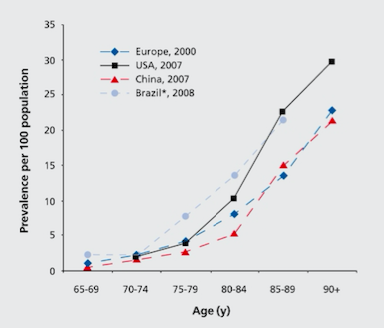
Globally, the prevalence of Alzheimer’s disease is estimated to be 24 million, and is predicted to double every 20 years (Mayeux & Stern, 2012). North America’s population is aging, and as a result of this, there is an exponential rise of patients diagnosed with Alzheimer’s disease as seen in Figure 1 (Mayeux & Stern, 2012). The highest prevalence of Alzheimer’s is in North America, Western Europe, Latin America, and China (Reitz, Brayne & Mayeux, 2011). The average age when patients are affected is at 60 years (Reitz et al., 2011). The annual incident rates (per 1000) in these countries are 10.5 in North America, 8.8 in Western Europe, 9.2 in Latin America, and 8.0 in China (Reitz et al., 2011).
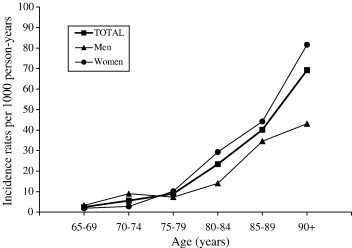
Studies have shown that although advancing age is a risk factor, there is also a sex difference in incidence and prevalence rates (Bermejo-Pareja, Benito-León, Vega, Medrona, & Román, 2008). Research shows that women have a higher risk of developing AD in the population older than 85 years of age (Figure 2) (Bermejo-Pareja et al., 2008). Incidence figures increase with age in women, but decreased beyond age 90 in men.
Etiology
Several risk factors have been suggested as causative agents for Alzheimer’s that are associated with the etiology and outcome of the disease such as:
- Cerebrovascular Disease - Changes such as hemorrhagic infarcts, small and large ischemic cortical infarcts, and white matter changes all increase the risk of dementia. Stroke can lead to cognitive impairment and Alzheimer’s as it can directly damage brain regions important in memory function such as thalamus. Stroke can also increase the amyloid-β deposition, which can lead to cognitive decline (Reitz et al., 2011)
- Hypertension - Increase risk of AD by decreasing the vascular integrity of the blood-brain barrier, which results in protein extravasation into brain tissue. This can lead to cell damage, reduction in neuronal or synaptic function, apoptosis, and an increase in amyloid-β deposition, resulting in cognitive impairment (Reitz et al., 2011)
- Smoking- Increases generation of free radicals, leading to high oxidative stress, which leads to activation of phagocytes and further oxidative damage. Smoking can also promote cerebrovascular disease, which is a risk factor for AD (Reitz et al., 2011)
Other risk factors include:
- Family History - Those who have a family member that has AD is more likely to develop the disease. Risk increases if more than one family member has the illness (Reitz et al., 2011).
- Genetics (Hereditary):
- Risk Genes - Increase likelihood of developing a disease. Research shows several genes that increase of AD such as the apolipoprotein E-e4 (APOE-e4) and this has the strong impact. If one copy of APOE-e4 is inherited, there is an increased risk of developing AD, and inheriting two copies results in an even higher risk (Reitz et al., 2011.
- Deterministic Genes- Directly causes a disease. Variations of the genes coding three proteins directly cause AD, and this includes amyloid precursor protein (APP), presenilin-1 (PS-1), and presenilin-2 (PS-2). When AD is caused by a deterministic variation, it is known as familial AD (Reitz et al., 2011).
Diagnosis
sMRI: Structural Magnetic Resonance imaging or sMRI for short, is a system of neuroimaging that provides static anatomical information of the brain (Symms, et. al, 2004). This is a non-invasive technique that can be used to diagnose neurodegenerative disorders as it allows to see the anatomy and pathology of the brain. It also allows doctors to see the appearance of the brain changing due to a specific pathophysiology. Moreover, structural imaging is a tool that is used in the diagnostic criteria for Alzheimer’s patients as it provides a better understanding of the structural changes that occur in the brains of patients (Frisoni, et. al., 2011). Structural markers can be used to differentiate the mild cognitive impairment stage to later progressions of the disorder (Frisoni, et. al., 2011). For example, sMRI provides an image of the progression of the amyloid plaques that are commonly associated with alzheimer's (Frisoni, et. al., 2011). sMRI can also show the tissue damage or loss of vital brain features as well (Frisoni, et. al., 2011). However, as a limitation, sMRI does not provide any information of the function of the brain, or any physiological brain activity (Symms, et. al, 2004).
fMRI:
Functional magnetic resonance imaging or fMRI is another non-invasive neuroimaging technique that helps detect changes in brain pattern and activities. It is widely used to better understand the changes in brain pathology of many disorders including Alzheimer's disease. It detects signal changes that are used to produce magnetic resonance imaging, implicating changes in neuronal brain activity (Gore, 2003). Specifically, fMRI detects blood oxygen dependant changes (BOLD) in the brain and uses it to visualize changes in neural brain activity (Gore, 2003). Therefore, fMRI provides functional information about the brain. It is also used to better understand functional changes in the brain of Alzheimer's patients. For example, fMRI images provides information on hippocampus alterations in patients with Alzheimer’s (Wang, et. al., 2005). Moreover, fMRIs show a decrease in brain activity when doing visual encoding tasks (Rombouts, et. al., 2005). It allows doctors to better understand changes in brain physiology patterns however, providing a clearer diagnosis. fMRI’s also allow to better compare the level of the disease and the functional brain patterns as well.
Fluro-deoxy-D-Glucose Positron Emission Tomography (FDG PET):
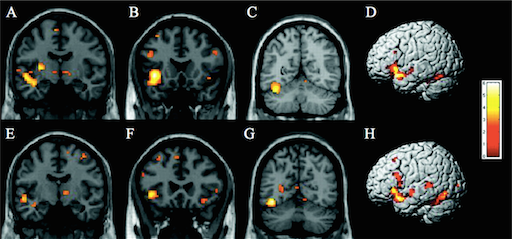
Flurodeoxyglucose is a glucose analog which is labeled with Fluorine-18 (half-life 110 min), and takes advantage of the brain’s primary fuel source in order to capture synaptic activity via PET scan (Johnson et al., 2012). Normal cerebral glucose metabolism has been established with aging, making changes that occur in early AD and throughout, precise through this type of PET scan (Marcus et al., 2014). FDG is a sufficient biomarker for overall brain metabolism as seen with evidence in a study by Rocher et al. in 2003, which illustrated that FDG uptake strongly correlated with levels of synaptic vesicle protein synaptophysin, found during autopsy. Over the years, many studies have proved the efficacy of recognizing FDG-PET scans to better aid with the diagnosis of Alzheimer’s. Along with a recognizable endophenotype for AD, there are resembling hypometabolic regions of the brain that are characteristic in established AD patients (Johnson et al., 2012). The metabolic deficiencies can be seen in Figure 3, showing various regions of the parietotemporal association cortices, posterior cingulate cortex, and the precuneus, reduced due to decreased synaptic activity, thus resembling the FDG-PET endophenotype (Johnson et al., 2012; Marcus et al., 2014). A meta analysis by Bloudek et al., 2011, showed that out of 119 modalities used to diagnose AD, FDG-PET had superior diagnostic capability as compared to MRI, CT, SPECT and biomarkers.
Figure three: A FDG-PET scan outlining the endophenotype typical of an Alzheimer’s patient in the mild to moderate stage of AD. The precuneus, lateral parietal, lateral temporal, and medial temporal lobes have slowly decreased in neuronal mass over time thus displaying decreased glucose metabolism.
Signs, Stages & Symptoms
Alzheimer’s disease is progressive and composed of different stages, characterized by different symptoms that also worsen over time (Alzheimer’s Disease, 2016).
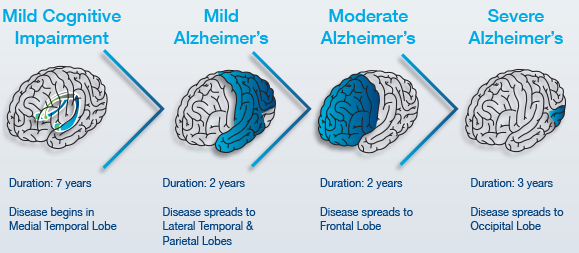
Pre-Clinical AD: Early Stage (7-20 years)
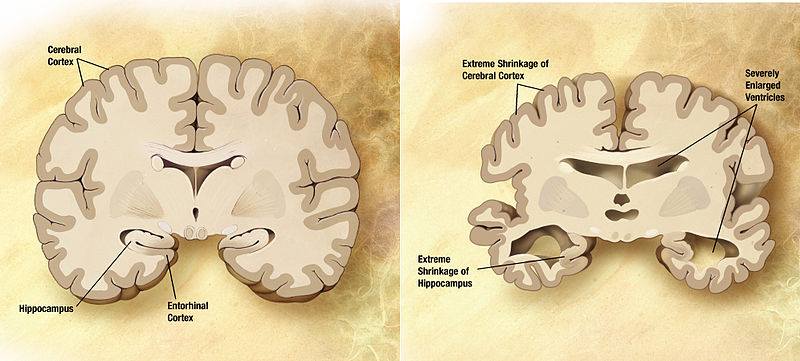
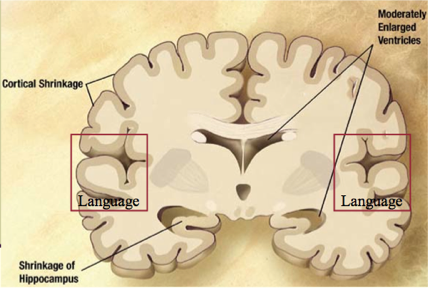
Changes may start to happen as long as twenty years or more before a clinical diagnosis, such as the slow formation of plaques and tangles causing short-term memory loss (Alzheimer’s Association, n.d). Also known as the pre-dementia stage or mild-cognitive impairment (MCI), patients may experience a hinderance in both learning new information and their ability to access the semantic memory system (Förstl & Kurz, 1999). As such, the patient may slowly forget familiar words, colours, material they just read, misplace valuable objects and have trouble planning and organizing (Alzheimer’s Association, n.d). Also at this stage, clinically, there is no differentiation between the early stages of Alzheimer’s Disease (AD) and other non-AD memory impairments that may be benign and/or reversible, causing unreliability in detecting and providing treatment as early as possible (Arlt, 2013). The brain areas affected in this stage can be seen in Figure 3 and are the entorhinal cortex and the hippocampus, in charge of thinking/planning, and learning/memory respectively. Some alternate distinctive changes that may be seen are short term memory loss and the shrinkage of the amygdala (Alzheimer’s Association, n.d).
Early Stage Symptoms:
- Short-term memory loss
- Language problems characterised by a decreased vocabulary and word fluency but patient with AD can communicate basic ideas adequately<br>
Mild to Moderate AD: Mid-Stage (2-4 years)
Also known as the mild to moderate dementia stage, most AD patients are diagnosed in this stage. There is an observed increase in plaques and tangles found in the hippocampus and frontal lobe, causing memory hinderance serious enough to interfere with work or social life (Alzheimer’s Association, n.d). Moreover, plaques and tangles will spread to the speaking/understanding speech as well as the spatial orientation areas of the brain, resulting in the shrinkage of the cortical area, shrinkage of hippocampus and moderately enlarged ventricles (Alzheimer’s Association, n.d). In the early parts of this stage, patients may be able to live independently, however as the disease progresses, patients tend to “live in the past”, language, reading and writing skills worsen and patients gradually lose insight into their own condition— so close supervision is necessary (Förstl & Kurz, 1999). As Alzheimer's progresses, individuals may experience changes in personality and behaviour as well, resulting in trouble recognizing family members (Alzheimer’s Association, n.d). Due to this, family support seems to diminish due to restlessness, aggression, disorientation and involuntary urinary excretion (Förstl & Kurz, 1999). At the later times of this stage, closer to the Severe Stage, the parietal, frontal and temporal lobes will all be affected with this disease.
Mid-Stage Symptoms:
- Memory loss continues
- Confusion of location of familiar place
- Taking longer to accomplish normal daily tasks
- Poor judgement
- Language impairment
- Mood changes
Severe AD: Late Stage (~3 years)
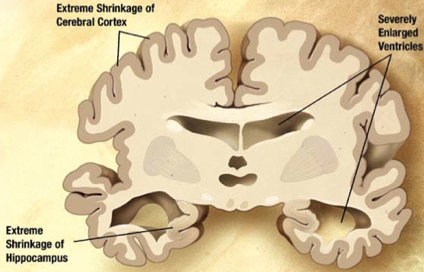
In this stage, almost all cognitive functions are severely impaired, while the brain has decreased in mass significantly due to widespread cell death (Alzheimer’s Association, n.d). Moreover, the extreme shrinkage particularly in the cerebral cortex and hippocampus is accompanied by a large increase in the ventricles of the brain (Alzheimer’s Association, n.d). The language of patients is severely reduced to simple phrases, however emotions can still be broadcasted or received (Förstl & Kurz, 1999). Nursing support is also disrupted due to many reasons. One being the patient's inability to understand nursing interventions, leading to angered experiences, along with displays of apathy and exhaustion (Förstl & Kurz, 1999). Also, due to impaired motor functions (chewing and swallowing) and large disturbances, nurses may have a harder time feeding AD patients (Förstl & Kurz, 1999). Moreover, plaques and tangles are widespread throughout the brain now, and severe dementia, and weight loss due to bed-riddance are often observed in the Severe AD stage (Alzheimer’s Association, n.d).
Late Stage Symptoms:
- Memory is completely lost
- Language is jumbled
- Autonomic functions are compromised
- Loss of mobility
- Weight loss
Pathophysiology
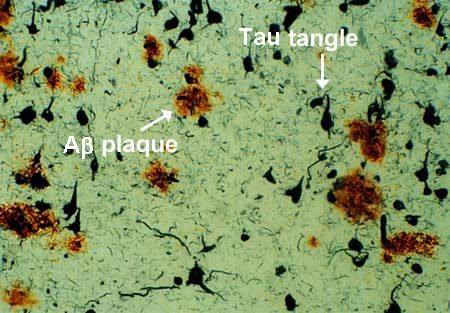
Alzheimer's disease is characterized by neuronal loss, decrease in cortical neurons and synapses in the brain. This results in the degeneration of an individual's cognitive capacity. From numerous studies, it was identified that amyloid plaques and Tau proteins are heavily concentrated in AD patient's brains. Thus, implying a significant involvement in the progression of AD (Ballatore et al., 2007).
Tau Proteins
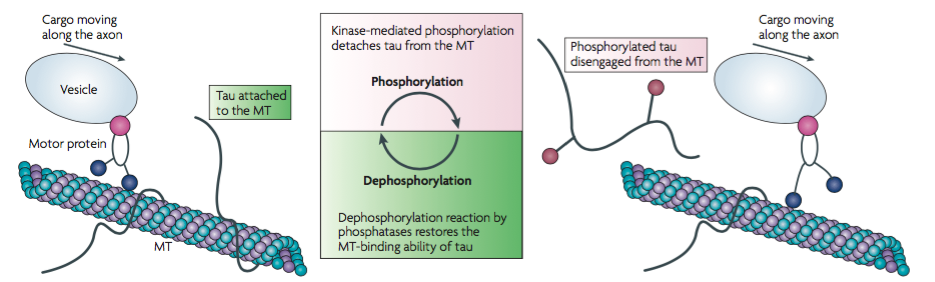
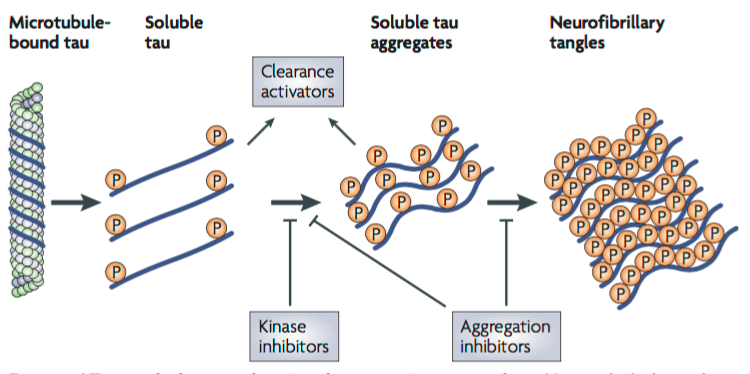
Tau proteins are microtubule-associated proteins (MAPs) found on the surface of microtubules in a neuronal cell. They stabilize the microtubules and prevent depolymerization to allow for the transportation of essential nutrients across the axon (Delacourte & Defossez, 1986). Tau proteins allow for normal axonal growth and maintenance of the internal structure of the neuronal transport system. Protein kinases such as GSK3 and CDK5 are involved in the phosphorylation of Tau proteins. The kinases cause Tau proteins to detach from the microtubules to allow cargo to move across the axon. After the cargo has safely transported across the axon, a dephosphorylation reaction occurs by which phosphatases reattach Tau proteins to the microtubules (Ballatore et al., 2007).
Hyperphosphorylation of Tau proteins results in the formation of neurofibrillary tangles (NFTs). When Tau proteins are hyperphosphorylated, they dissociate from microtubules, thus destabilizing them (Ballatore et al., 2007). The structure of the microtubules deteriorates such that they can no longer actively participate in the neuronal transport system. Free roaming Tau proteins aggregate to form NFTs which usually begin to form at the temporal lobe of the brain. Tau proteins can no longer be dephosphorylated to reattach to the degraded microtubules and the neuronal cell is ultimately destroyed (Ballatore et al., 2007). Research shows that the location and density of NFTs play a critical role in the pathophysiology and progression of AD. The direction of progression can vary and thus initial symptoms of AD vary between individuals (Ballatore et al., 2007).
Amyloid Precursor Protein
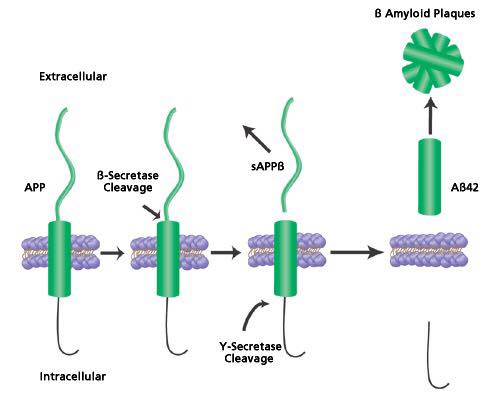
The amyloid precursor protein is a large membrane protein found on the surface of cells and is said to be associated with the growth and repair of neural cells (Goodsell, 2006). It is made up of four domains, three of which that can extend on the surface of cells and one peptide that expands the length of the entire membrane (Goodsell, 2006). As an intact protein, the APP can act as a G protein receptor and can bind to other structural molecules such as heparin and laminin (Goodsell, 2006). The APP can also be broken down by proteases known as secretases into small and large pieces (Goodsell, 2006). The larger pieces help with neural growth once released to the outside surface. On the other hand, the smaller pieces have also been implicated with Alzheimer's disease (Goodsell, 2006).
Histopathological features of the Alzheimer’s brain: The formation of plaques from the proteolytic cleavage of the APP protein has played a central role in the pathogenesis of Alzheimer’s disease (Janus, et. al, 2000). The plaques are formed from 40-42 amino acid B-amyloid peptide chains and are found on the surface of the cells (Roberds, et. al, 2001). This AB peptide is cleaved from the APP protein via secretase enzymes such as the beta secretase which cleaves the APP from the N terminus. Moreover, spinal fluid concentrations of patients with Alzheimer’s show anywhere between 500-900ng/ml of AB plaques (Seeman and Seeman, 2011). This concentration is generally much lower in the plasma suggesting the presence of receptors on neurons and glial cells (Seeman and Seeman, 2011). These are suggested to be damaging to the neurons and glial cells (Seeman and Seeman, 2011). Moreover, the presence of B-amyloid are correlated with the formation of plaques (Seeman and Seeman, 2011) since single molecules of Beta amyloid tend to form dimers and trimers, leading to the formation of plaques (Seeman and Seeman, 2011).
APP mutation in Familial Alzheimer's disease: Familial Alzheimer's disease is implicated with an early onset of alzheimer's which occurs before the age of 65. The pathology of early onset Alzheimer's is associated with the cerebral deposition of amyloid peptides in early onset of Alzheimer's (Vassar, et. al, 1999). These AB peptides are formed upon cleavage of the APP protein by two proteases - the Beta and gamma secretase (Vassar, et. al, 1999). One study looked at the overexpression of the protease BACE (Beta site APP cleaving enzyme) (Vassar, et. al, 1999). The results of the study showed an overexpression of B-secretase which has shown to be responsible for the cleavage of AB plaques in the cerebellum (Vassar, et. al, 1999).
Therapeutics for Alzheimer’s Disease
The copious complex mechanisms involved in the pathogenesis and pathophysiology of Alzheimer’s Disease (AD) creates a challenge to innovate effective therapeutics to delay or halt the disease progression (Fig.1) (Anand, 2014). However, extensive scientific efforts have been devoted into pharmacotherapeutic research for the treatment of AD. The development of present therapeutics have been based upon the first theory proposed to explain the disease known as the cholinergic hypothesis (Francis, 1999). This study evidently reveals the selective loss of cholinergic neurons in the nucleus basalis, which results in reduction in cholinergic activity among patients with AD. (Whitehouse et al., 1981). Additional studies confirm that AD may be associated with diminution of cholinergic activity, further studies utilized rhesus monkeys to show the effects of anticholinergic scopolamine on memory deficits as seen in AD (Bartus, 1978). Therefore, the development of therapeutics to augment cholinergic activity such as cholinesterase inhibitors (CIs) have primarily been focused upon. The CI’s function to enhance the cholinergic transmission by reducing in the breakdown of acetylcholine (ACh). For this reason, elevated levels of ACh are presented between neuronal synaptic clefts, thereby, compensating for the attenuated levels of ACh due to cholinergic neuronal death (Stahl, 2000). Currently, four Cis for symptomatic treatment of the disease have been approved by the FDA – donepezil, rivastigmine, galantamine, tacrine (Lleo, 2007). Such therapies have been recognized as first-line of treatment for mild to moderate progression of AD, though, tacrine is no longer used in clinic due to its associated effects of tacrine-induced liver damage (Alfirevic, 2007). A systematic review and metanalysis conducted by Hansen et al. has shown that donepezil, galantamine, and rivastigmine have shown overall benefits for stabilizing or slowing decline in cognition, function, behavior and clinical global change (Hansen, 2008). Despite the benefits of CI for moderate AD, the elevation in the levels of ACh can result in cholinergic adverse effects among patients which include nausea, vomiting, diarrhea, bradycardia, muscle cramps, and insomnia (Ellis, 2005). Moreover, memantine – an N-methyl D-aspartate (NMDA) receptor antagonist provides a treatment option for moderate to severe cases of AD (Yiannopoulou, 2013). Such drug serves to preclude the excessive release of an excitatory neurotransmitter glutamate resulting in excitotoxicity (Aprahamian, 2013). Moreover, a study by McShane et al. shows the benefit of memantine in moderate to severe to severe AD, which includes improvement on cognition, activities of daily living, and behavior at six months (McShane, 2006). However, although memantine is shown to be well tolerated in clinical studies, it can commonly be associated with side effects such as dizziness, constipation, confusion, headaches, hypertension, comnolence and visual hallucinations (McShane, 2006; Gauthier, 2006 ). While CI’s have been used as a standard mode of treatment to offer palliative care, ongoing research has been conducted for treatments capable of halting or modifying the progression of AD (Yiannopoulou, 2013).
Aβ Peptide Immunization
A study conducted by Janus and colleagues looked at the effects of immunization of AB peptides in murine models of Alzheimer’s disease (Janus, et. al, 2000). Evidence suggests that there was a reduction in amyloid plaques, reducing cognitive dysfunction in the murine models (Janus, et. al, 2000). The team began by injecting a mutated APP transgene in the murine models. For three months, the mices cognitive function and brain physiology were monitored showing both spatial deficits and an increase in the amount of AB plaques (Janus, et. al, 2000). The mice were vaccinated with AB42 or an islet-associated polypeptide known as IAPP at different time frames (Janus, et. al, 2000). After vaccination, the mice were tested in spatial memory using the Morris water maze test (Janus, et. al, 2000). AB42 immunized mice on average performed better than IAPP immunized mice(Janus, et. al, 2000). Moreover, there was a 50% reduction in AB plaques in the mice vaccinated by the AB42 vaccination(Janus, et. al, 2000).
BACE Inhibition
The gene that encodes the beta-secretase, a popular protease of the APP protein, is known as the BACE protein (Roberds, et. al, 2001). Inhibiting the production of BACE is seen to reduce the levels of beta-secretase (Vassar, et. al, 1999). Future research is looking at the development of BACE inhibitors to reduce the onset of familial Alzheimer's (Vassar, et. al, 1999). For example, BACE knockout studies in mice show a reduction in beta-amylase production (Roberds, et. al, 2001). Moreover, knocking out BACE does not have any harmful physiological effects of phenotypic differences in the mice, indicating future therapeutics.
Future Research & Implications
Targeting Tau Aggregates
Tau is a soluble microtubule-binding protein. One of the functions of Tau is to stabilize microtubules in axons for axonal transport, and as cytoskeletal elements for growth (Citron, 2010). One of the characteristics observed in AD neurons consist of hyperphosphorylated, aggregated insoluble tau (Citron, 2010). This leads to direct toxic effects such as a loss of axonal transport as tau can be detached from microtubules leading to the formation of soluble tau aggregates forming neurofibrillary tangles (Citron, 2010). Current therapeutic strategies focus on the inhibition of tau aggregation, and to block tau hyperphosphorylation (Citron, 2010). One of these strategies is to design kinase inhibitors, which would prevent hyperphosphorylation, and design aggregation inhibitors that would block the soluble tau aggregates and formation of tangles (Citron, 2010). Tau toxicity can also be prevented by enhancing clearance of tau, and degradation of tau aggregates (Citron, 2010).
Targeting Aβ Plaques
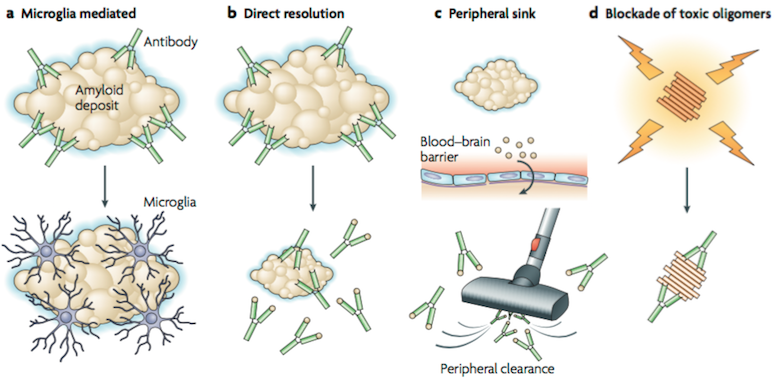
Over the past few years, amyloid-β (Aβ) immunotherapy have become a fascinating area of research in AD. Research in this field was initiated after the publication of the first immunization paper from Elan that reported that amyloid pathology was reduced in an APP transgenic mouse model after vaccination with aggregated Aβ (Citron, 2010). Three hypotheses have been proposed regarding Aβ immunotherapy mechanism. The first mechanism (Figure A) is based on microglial activation and phagocytosis. In this mechanism, amyloid-specific antibodies are administered and reach the central nervous system, bind to amyloid deposits (plaque), and trigger microglia to phaocytose the amyloid (Citron, 2010). The second mechanism (Figure B) is a direct interact interaction of amyloid-specific antibodies with the amyloid deposits. The antibodies are able to resolve the in vitro aggregated Aβ, however research is still being done on how the amounts of antibody administered can dissolve the existing insoluble fibrils in the brain (Citron, 2010). A follow-up mechanism was proposed, in which peripheral amyloid-specific antibodies act as a sink (Figure C), and pull soluble Aβ into periphery where it is cleared (Citron, 2010). In vivo studies identified an efficient receptor-mediated transport mechanism for Aβ at the blood brain barrier, where Aβ is transported from CNS to plasma, and from plasma to CNS (Demattos, Bales, Cummins, Dodart, Paul & Holtzman, 2001). Research data suggests that to alter the CNS Aβ levels, increase efflux of Aβ from CNS to plasma and/or decrease efflux of Aβ from plasma to CNS is needed (Demattos et al., 2001). The experiment demonstrated that the Aβ monoclonal antibody 266 (m266) showed affinity to soluble Aβ, and did not bind to plaques (Demattos et al., 2001). This reduced the amyloid levels upon administration. It was concluded that sufficient antibody concentrations were required to produce noticeable levels of cerebrospinal fluid capture needed to capture soluble Aβ, and produce a net flux of Aβ from the CNS to periphery, leading to decreased amyloid levels (Citron, 2010). Although peripheral administration of m266 reduced Aβ deposition, m266 did not bind to the deposits (Demattos et al., 2001). Hence, m266 appears to reduce brain Aβ burden by altering the CNS and plasma Aβ clearance (Demattos et al., 2001).
Conclusion
Alzheimer’s disease is a very common form of dementia that has significant implications to future health research, and well-being of elderly. There are two cases of Alzheimer’s disease, sporadic and familial, with sporadic being the most common and associated with late-onset Alzheimer’s. Sporadic cases occur because of the common variant allele, E4 of the apolipoprotein altering the normal functions, and resulting in amyloid-beta aggregation. Epidemiology research shows that age is one of the risk factors for Alzheimer’s, but Alzheimer’s is not a normal part of aging. There are many symptoms, which can all be attributed to a loss of cognitive function, and memory decline, and these symptoms progress through the various stages of Alzheimer’s : early, mild-moderate, severe. This puzzling and debilitating disease has no forms of prevention and cure. The treatments for Alzheimer’s that are offered are only able to alleviate the symptoms and slow the progression, but do not cure the disease completely. Future therapeutics are focusing on more specific ways to target tau tangles, and amyloid-beta plaques in a more effective manner as these two have been found to be the indicator of Alzheimer’s in most cases.
References
Alfirevic, A., Mills, T., Carr, D., Barratt, B. J., Jawaid, A., Sherwood, J., … Pirmohamed, M. (2007). Tacrine-induced liver damage: an analysis of 19 candidate genes. Pharmacogenetics and Genomics, 17(12), 1091–100. https://doi.org/10.1097/FPC.0b013e3282f1f12b
Alzheimer’s Association. (n.d). Stages of Alzheimer’s & Symptoms. Retreived October 26, 2017, from https://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp?type=brainTourFooter#overview
Alzheimer's Disease and Related Dementias. (2016, August). Retrieved October 23, 2017, from https://www.nia.nih.gov/health/alzheimer
Anand, R., Gill, K. D., & Mahdi, A. A. (2014). Therapeutics of Alzheimer’s Disease: Past, Present and Future. Neuropharmacology, 76, 27–50. https://doi.org/10.1016/j.neuropharm.2013.07.004
Aprahamian, I., Stella, F., & Forlenza, O. V. (2013). New treatment strategies for Alzheimer’s disease: is there a hope? Indian J Med Res, 138(4), 449–460. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24434253
Arlt, S. (2013). Non-alzheimer’s disease- related impairment and dementia. Dialougues Clin Neurosci, 15, 4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3898684/
Ballatore, C., Lee, V. M. Y., & Trojanowski, J. Q. (2007). Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Reviews Neuroscience, 8(9), 663-672.
Bartus, R. T. (1978). Evidence for a direct cholinergic involvement in the scopolamine-induced amnesia in monkeys: Effects of concurrent administration of physostigmine and methylphenidate with scopolamine. Pharmacology, Biochemistry and Behavior, 9(6), 833–836. https://doi.org/10.1016/0091-3057(78)90364-7
Bermejo-Pareja, F., Benito-León, J., Vega, S., Medrano, M., & Román, G. (2008). Incidence and subtypes of dementia in three elderly populations of central Spain. Journal of the Neurological Sciences, 264(1-2), 63-72. doi:10.1016/j.jns.2007.07.021
Bloudek L.M., Spackman D.E., Blankenburg M., & Sullivan, S.D. (2011). Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis, 26, 627–645.
Citron, M. (2010). Alzheimer's disease: strategies for disease modification. Nature reviews Drug discovery, 9(5), 387-398.
Clement, F., & Belleville, S. (2009). Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum Brain Mapp, 30, 4033–4047
Delacourte, A., & Defossez, A. (1986). Alzheimer's disease: Tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. Journal of the neurological sciences, 76(2), 173-186.
DeMattos, R. B., Bales, K. R., Cummins, D. J., Dodart, J.-C., Paul, S. M., & Holtzman, D. M. (2001). Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8850–8855. http://doi.org/10.1073/pnas.151261398
Ellis, J. M. (2005). Cholinesterase inhibitors in the treatment of dementia. The Journal of the American Osteopathic Association, 105(3), 145–158. https://doi.org/10.7556/JAOA.2005.105.3.145
Förstl, H. & Kurz, A. (1999). Clinical features of alzheimers disease. Eur Arch Psychiatry Clin Neurosci, 249, 288-290. https://doi.org/10.1007/s004060050101
Francis, P. T., Palmer, A. M., Snape, M., & Wilcock, G. K. (1999). The cholinergic hypothesis of Alzheimer’s disease: a review of progress. Journal of Neurology, Neurosurgery, and Psychiatry, 66(2), 137–47. https://doi.org/10.1136/jnnp.66.2.137
Frisoni, G. B., Fox, N. C., Jack, C. R., Scheltens, P., & Thompson, P. M. (2010). The clinical use of structural MRI in Alzheimer disease. Nature Reviews Neurology, 6(2), 67-77.
Gauthier, S., Herrmann, N., Ferreri, F., & Agbokou, C. (2006, August 29). Use of memantine to treat Alzheimer’s disease [1]. CMAJ. https://doi.org/10.1503/cmaj.1060168
Gore, J. C. (2003). Principles and practice of functional MRI of the human brain. Journal of Clinical Investigation, 112(1), 4.
Hansen, R. A., Gartlehner, G., Webb, A. P., Morgan, L. C., Moore, C. G., & Jonas, D. E. (2008). Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interventions Aging, 3(2), 211–225.
Hippius, H., & Neundörfer, G. (2003). The discovery of Alzheimer's disease. Dialogues in clinical neuroscience, 5(1), 101.
Johnson, K. A., Fox, N. C., Sperling, R. A., & Klunk, W. E. (2012). Brain Imaging in Alzheimer Disease. Cold Spring Harb Perspect Med, 2(4), a006213. http://doi.org/10.1101/cshperspect.a006213
Lleó, A. (2007). Current therapeutic options for Alzheimer’s disease. Current Genomics, 8(8), 550–8. https://doi.org/10.2174/138920207783769549
Marcus, C., Mena, E., & Subramaniam, R. M. (2014). Brain PET in the Diagnosis of Alzheimer’s Disease. Clin Nucl Med, 39(10), e413–e426. http://doi.org/10.1097/RLU.0000000000000547
Mayeux, R., & Stern, Y. (2012). Epidemiology of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine, 2(8), 10.1101/cshperspect.a006239 a006239. http://doi.org/10.1101/cshperspect.a006239
Putcha D, O’Keefe K, LaViolette P, O’Brien J, Greve D, Rentz D, Locascio JJ, Atri A, Sperling R (2010). Reliability of fMRI associative encoding memory paradigm in non-demented elderly adults. Hum Brain Mapp, 32(12), 2027-44.
Reitz, C., Brayne, C., & Mayeux, R. (2011). Epidemiology of Alzheimer disease. Nature Reviews. Neurology, 7(3), 137–152. http://doi.org/10.1038/nrneurol.2011.2
Rocher, A.B., Chapon, F., Blaizot, X., Baron, J.C., Chavoix, C. (2003). Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: A study in baboons. Neuroimage 20, 1894–1898
Stahl, S. M. (2000). The new cholinesterase inhibitors for Alzheimer’s disease, part 2: Illustrating their mechanisms of action. Journal of Clinical Psychiatry.
Stam, C. J. (2014). Modern network science of neurological disorders. Nature Reviews Neuroscience, 15(10), 683-695.
Symms, M., Jäger, H. R., Schmierer, K., & Yousry, T. A. (2004). A review of structural magnetic resonance neuroimaging. Journal of Neurology, Neurosurgery & Psychiatry, 75(9), 1235-1244.
Vemuri, P., & Jack, C. R. (2010). Role of structural MRI in Alzheimer's disease. Alzheimer's research & therapy, 2(4), 23.
Wang, L., Zang, Y., He, Y., Liang, M., Zhang, X., Tian, L., … & Li, K. (2006). Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage, 31(2), 496-504.
Yiannopoulou, K. G., & Papageorgiou, S. G. (2013). Current and future treatments for Alzheimer’s disease. Therapeutic Advances in Neurological Disorders, 6(1), 19–33. https://doi.org/10.1177/1756285612461679