Table of Contents
Alzheimer's Disease
Origin and Background
In 1906, a German psychiatrist and neuropathologist by the name of Alois Alzheimer was drawn to the case of a woman showing unusual symptoms. Post mortem analysis of the brain tissue of the woman started the journey of Dr. Alzheimer’s description of the neurodegenerative disease (Hippius, 2013). Alzheimer disease is an incurable disorder of cognitive and behavioural impairment with a long and progressive timeline. 75% of patients with dementia are thought to have genetically based Alzheimer’s, however the incidence can also be sporadic (Chapman et al, 2006). In Alzheimer’s, misfolded amino acid aggregates known as plaques develop in brain areas and impact and impede neuronal communication causing brain cell atrophy. This neurodegeneration begins in the hippocampus, then spreads to the rest of the brain over time and increasing severity of the disease. The affected region of the brain is the reduction of the memory encoding hippocampus, along with associated regions of the cerebral cortex involved in thinking, decision making, and planning (Purohit et al, 1998)
Epidemiology
46 million people live with dementia worldwide, and most of these cases are attributed to Alzheimer’s disease. This number is expected to increase due to the elderly being the most rapidly growing portion of Canada and US populations (Abbott, 2011). As the population ages, Alzheimer’s disease is expected to become increasingly prevalent. Within the next 25 years, the number of people with Alzheimer’s disease in the US will triple (Kandel et al, 2013). It is important that enough research is being devoted to Alzheimer’s disease. Most funding is allocated to other research related to the main causes of death, but because the course of Alzheimer’s is between 7-10+ years whereas cancer for example is about 4 months, there are less deaths compared to cancer and this may be overridden (Leading causes of death, by sex (Both sexes), 2015). Due to the longer course, dementia and thus Alzheimer’s disease incurs more costs, even though there is not as much investment compared to other leading causes of death (Abbott, 2011).
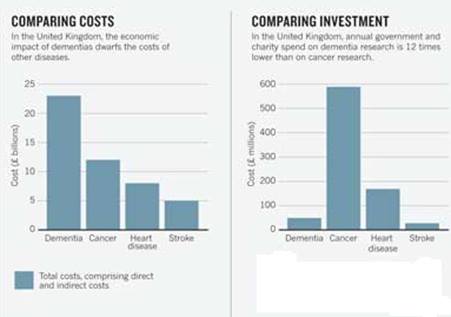
Figure 1:This figure shows that dementia has the largest economic burden in the UK, but receives the lowest funding. (Abbott, 2011).
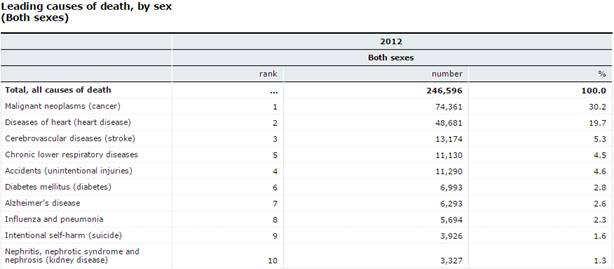
Figure 2:This figure shows the leading causes of death by sex (2015). Since Alzheimer's disease is not one of the leading causes of death due to its long course, not as much funding is allocated to it (Statistics Canada Leading causes of death, by sex (Both sexes) 2015).
Symptoms
At first, language, strength, reflexes, sensory capacities and motor skills are spared. Memory loss increases as the disease progresses, often leading to confusion. Cognitive abilities such as problem solving, language, calculation and visuospatial skills are compromised with progression of the disease (Kandel et al, 2013). Due to the loss of cognitive abilities, experiencing psychotic symptoms are common such as hallucinations and delusions. Mental functions and the ability to carry out usual daily activities become impaired (Kandel et al, 2013). The late stages of Alzheimer's are related to becoming mute, loss of autonomic functions and eventually becoming bedridden, relying completely on a caregiver (Kandel et al, 2013). Because shrinkage of the amygdala is also a hallmark of Alzheimer’s, it is associated with mood changes.
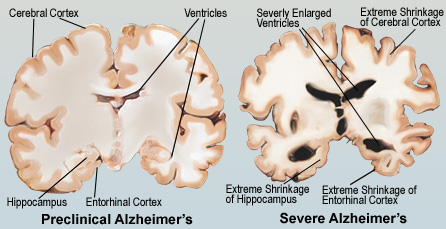
Figure 3:This figure illustrates the comparison between a pre-clinical and severe Alzheimer's brain (Neergaard, 2016).
Disease Progression
Mild: Patients with Alzheimer's may show physiological changes in the brain 10- 20 years prior to showing any symptoms. Shrinkage of the amygdala brings about symptoms of emotional outbursts and mood changes (Purohit et al, 1998)
Moderate: Memory loss at this stage advances to confusion of location in familiar places and poor judgement, mood changes, increased anxiety and language impairment (Hippius, 2013).
Severe: Plaques and tangles become widespread, memory loss advances to severe dementia and atrophy of other brain regions leads to progressive loss of autonomic functions (Hippius, 2013).
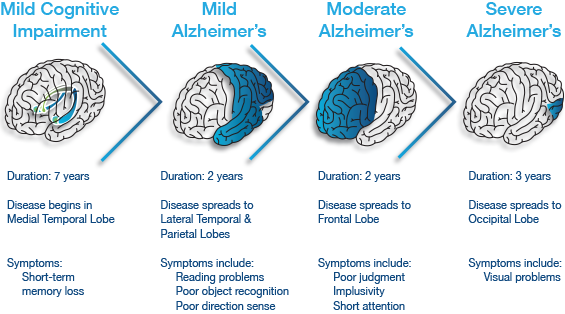
Figure 4:This figure illustrates the progressive impact of the neurodegenerative disease.
Etiology
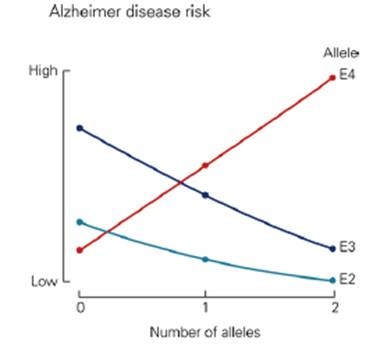
Sporadic cases: The greatest risk factor for Alzheimer’s is aging, but Alzheimer’s is not a normal part of aging. The most common form of Alzheimer’s disease is called sporadic Alzheimer’s disease, meaning it has no specific family link (Understanding Genetics and Alzheimer's Disease, 2016). Sporadic Alzheimer’s is associated with late-onset Alzheimer’s disease, which includes individuals older than 60 years of age. A certain form of a protein called apolipoprotein E (ApoE), has been found to increase the risk for sporadic Alzheimer’s (Kandel et al, 2013). The specific allele of the ApoE gene is ApoE4. This gene is expressed as three alleles, ApoE2, ApoE3, and ApoE4 (Kandel et al, 2013). The normal function of the ApoE protein is to carry cholesterol and other lipids in the blood. It also plays a fundamental role in the maintenance and repair of neurons, and it binds amyloid beta (Mahley, 2006). However, isoforms may bind amyloid beta differently, which may influence aggregation, deposition and clearance of amyloid beta (Kandel et al, 2013). In this case, ApoE4 is the poorest at binding to and clearing amyloid beta from the bloodstream, so amyloid beta continues to aggregate and the cascade of events, including neuronal injury, follows (Kandel et al, 2013). The exact mechanism through which ApoE4 works is unknown, but some studies have shown features of Alzheimer's pathology in transgenic mice expressing the human ApoE4 allele in neurons (Mahley, 2006). The features included reduced numbers of presynaptic terminals, increased phosphorylation of tau, increased plaque deposition and impaired learning and memory, found by testing mice in a water maze (Mahley, 2006).
Figure 5:This graph shows that the ApoE4 allele is a risk factor for sporadic Alzheimer's disease. It appears that ApoE2 and ApoE3 lower the risk for Alzheimer's disease (Kandel et al, 2013).
Familial Cases The familial, or inherited form of Alzheimer’s is associated with early-onset Alzheimer’s, which includes individuals 60 years of age or younger. Mutations in three genes are associated with this form of Alzheimer’s; APP, presinilin-1 and presinilin-2 (Kandel et al, 2013). The mutations influence the cleavage of APP, increasing the production of amyloid beta peptides, or increasing the ratio of the more toxic amyloid beta 42 kind. Some APP mutations are amino acid substitutions within the amyloid beta region (Kandel et al, 2013). Cells with this mutation secrete more amyloid beta peptides compared to wildtype APP. Another APP mutation influences gamma-secretase to selectively produce amyloid beta 42 instead of amyloid beta 40 (Kandel et al, 2013). The presinilin mutants are similar, as they produce mutant gamma-secretase as well, causing over activity of gamma-secretase, and again generating a higher ratio of amyloid beta 42 (Kanel et al, 2013). The mutations in APP or presinilin genes lead to a gain of function for overproducing amyloid beta 42.
Pathophysiology
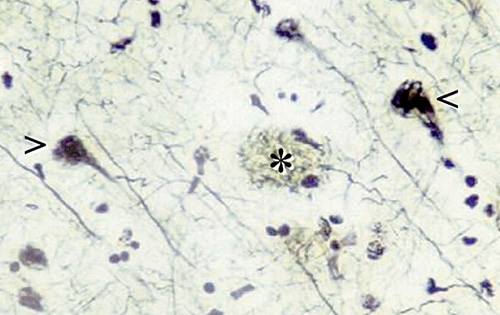
Dementia is defined as a group of symptoms including memory loss and other mental abilities. Investigators have learned that Alzheimer's disease is the most common causes of dementia. It accounts for about 60 - 80% of all dementia related cases (Seeman & Seeman, 2011). One of the prominent features of Alzheimer's is the loss of connections between cells. This results in diminished cell function or cell death. At this point, no one knows what exactly causes Alzheimer's disease but it appears that the damage to the brain starts at least 10 years before the issues become obvious. The classic biological hallmarks of the disease are β-amyloid plaques and neurofibrillary tangles, which are made of misfolded proteins (Seeman & Seeman, 2011).
Figure 6: The histopathological features of the Alzheimer's Brain, where the * indicates plaques and the < indicates tangles (Del Cerro & C. Triarhou, 2006).
β-Amyloid Plaques
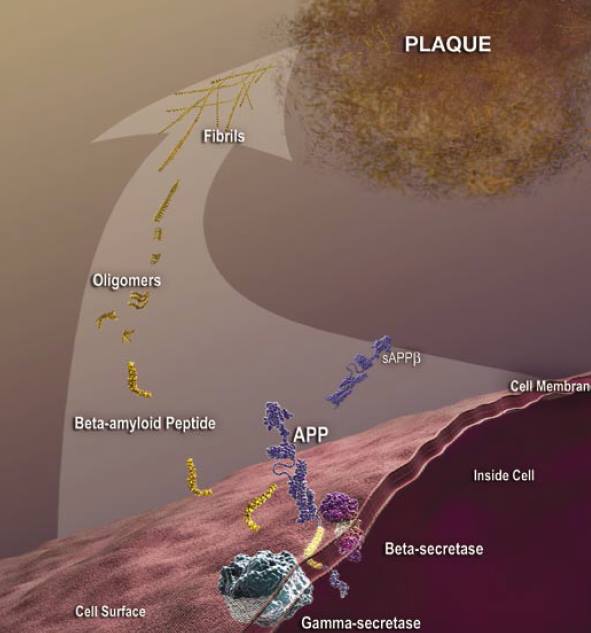
In the cell membrane the amyloid precursor protein (APP) helps neurons grow and repair themselves. Scientists have identified 3 enzymes responsible for cleaving APP (Lieff, 2015). These enzymes are called α-secretase, γ-secretase and β-secretase. Normally, APP is sliced by a α-secretase to generate a sliced-APP (sAPP) and a C83 carboxy-terminal fragment. The presence of sAPP is associated with normal synaptic signaling and result in synaptic plasticity, learning and memory, emotional behaviours and neuronal survival (Lieff, 2015). A small APP fragment remains tethered to the membrane but is later cleaved by γ-secretase. It is then released into the space outside the neuron where it eventually dissolves (Lieff, 2015). The brain of an individual with Alzheimer's disease follows a much different path. β-secretase replaces α-secretase, which then teams up with γ-secretase to cause a mutation that disrupts the function of APP. This mutation does not allow the β-amyloid peptide to breakdown resulting in small, soluble aggregates of two, three, four, or even up to a dozen beta-amyloid peptides called oligomers (Lieff, 2015). It is likely that some oligomers are cleared from the brain. Those that cannot be cleared clump together with more beta-amyloid peptides. As the process continues, oligomers grow larger, becoming entities called protofibrils and fibrils. Eventually, other proteins and cellular material are added, and these increasingly insoluble entities combine to become the well-known β-amyloid plaques (Lieff, 2015).
Figure 7: An animated image depicting the harmful pathway that results in β-amyloid plaques for individuals with Alzheimer's disease (Patterson et al., 2008).
Neurofibrillary Tangles
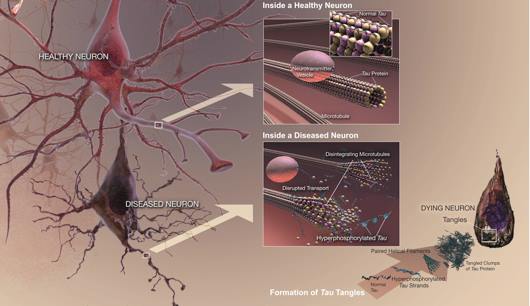
Tangles are abnormal collections of twisted protein threads found inside nerve cells. The main component of tangles is a protein called tau. Healthy neurons are internally supported in part by structures called microtubules, which help transport nutrients and other cellular components, such as neurotransmitter containing vesicles, from the cell body down the axon (Lieff, 2015). Tau, which usually has a certain number of phosphate molecules attached to it, binds to microtubules and appears to stabilize them. The brain of an individual with Alzheimer's disease has an abnormally large number of additional phosphate molecules attach to tau resulting in hyperphosphorylation. GSK-3 and CDK5 are the kinases primarily responsible for phosphorylation of Tau (PKC, PKA and Erk2 are also involved) (Lieff, 2015). The hyperphosphorylation of tau protein results in microtubule destabilization that disrupts the transportation of motor proteins. The disengaged tau tangle with the other tau proteins resulting in neurofibrillary tangles. Current research suggests that the beta amyloid plaques that build up outside the neuron initiates pathways inside the neuron, which leads to the hyperphosphorylation of tau (Lieff, 2015).
Figure 8: An animated image depicting the harmful pathway that results in neurofibrillary tangles for individuals with Alzheimer's disease (Patterson et al., 2008).
Diagnosis
It is suggested that the pathophysiological process of Alzheimer’s disease (AD) begins about 10-15 years before the diagnosis of clinical dementia (Sperling et al, 2011). Diagnosing AD in the early stages is difficult because the initial symptoms such as memory impairment and neuronal loss are part of normal age-related cognitive decline. Diagnosis of AD can only be confirmed with an autopsy by examining brain tissues (Kandel et al, 2013). Studies using new neuroimaging techniques and fluid biomarkers suggest that Alzheimer's disease pathology can be detected preclinically using different techniques. Amyloid plaques and neurofibrillary tangles are hallmarks of AD (Kandel et al, 2013). This also includes inflammation and neuronal, axonal and synaptic loss and dysfunction and ultimately neuronal death. However, these changes in the brain are seen before any cognitive declines (Kandel et al, 2013).
Figure 9: Plaques, neurofibrillary tangles, synaptic and neuronal loss appear in the brain prior to significant cognitive decline (Abbott, 2011).
Technique 1: Physical, neurological and neuropsychological examination
This technique measures motor and neurological skills to see if there is a cognitive decline. The problem with testing psychological traits is that there is nothing to compare these traits to (Kandel et al, 2013). It unknown whether this is a recent decline in memory or not. Memory decline is normal with old age, so a longitudinal study would be needed to compare the performance of an individual (Kandel et al, 2013).
Technique 2- MRI and fMRI
An MRI is used to view structural changes such as enlargement of cerebral ventricles, cortical thinning, and shrinkage of hippocampus and medial temporal lobe (Kandel et al, 2013). Functional magnetic resonance imaging (fMRI) is believed to provide a measure of synaptic activity, and this technique appears to show hypoactivation of the medial temporal lobe memory system in the clinical stages of the disease (Perrin et al, 2009). Due to neuronal loss, many other cortical regions also show evidence of decreased glucose metabolism in patients with Alzheimer’s (Perrin et al, 2009).
Technique 3- PET
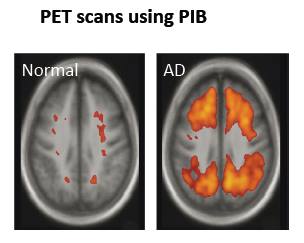
Amyloid plaques can be visualized using Positron Emission Tomography (PET) using a new radioactive compound called Pittsburgh compound B (PIB) that binds with high affinity to amyloid beta or tau (Kandel et al, 2013). PIB is injected into the bloodstream. In Alzheimer’s, PIB is retained specifically in areas with amyloid deposition (Kandel et al, 2013). Other PET labelling agents have been developed to image inflammation as reflected by activated microglia and reactive astrocytes that surround plaques (Kandel et al, 2013).
Figure 10:This image clearly shows PIB retained in brain areas associated with amyloid deposition. There is a lot more PIB in the brain on the right, the Alzheimer's brain, compared with the healthy brain on the left (Kandel et al, 2013).
Technique 4- Fluid Biomarkers
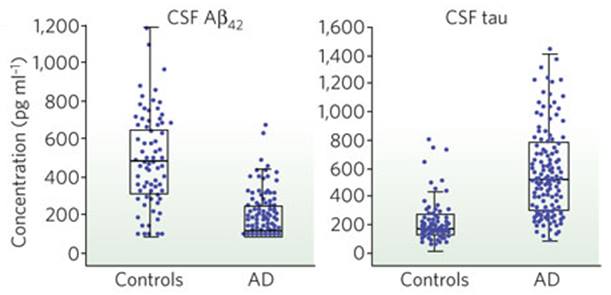
Longitudinal measurements of the levels of amyloid beta 42 rather than amyloid beta 40 are used because amyloid 42 is more hydrophobic and more prone to fibril formation and thus is the predominant form found in cerebral plaques (Kandel et al, 2013). This technique also looks at concentration of tau. This may allow clinicians to monitor and possibly even predict the progression of Alzheimer’s throughout its course (Kandel et al, 2013). In Alzheimer’s, it is expected that amyloid beta 42 levels will be lower in the cerebrospinal fluid versus the control (Kandel et al, 2013). This is due to the fact that amyloid beta 42 aggregates more easily and is cleared poorly, thus less amyloid beta 42 will be found in the cerebrospinal fluid. Tau is also a good biomarker because it is normally found in the intracellular fluid compartment of a neuron (Kandel et al, 2013). In Alzheimer’s, high concentrations of tau are found in the cerebrospinal fluid. This is due to the fact that when cells break apart, the plasma membrane breaks and thus tau is released into cerebrospinal fluid from neuronal injury and death (Kandel et al, 2013).
Figure 11: Using fluid biomarkers as a method to detect Alzheimer's. On the left, there are lower concentrations of amyloid beta 42 in cerebrospinal fluid compared to the control due to the aggregation of amyloid beta 42. On the right, there are higher concentrations of tau in the cerebrospinal fluid compared to the control, due to the cell death releasing tau (Kandel et al, 2013).
Treatments
Currently Alzheimer’s has no cure but there are current treatments available that can slow down the worsening of the symptoms and as a result improving individual’s quality of life.
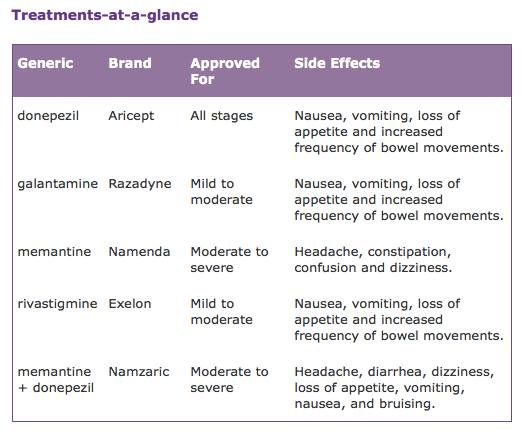
Figure 12: Table showing treatments according to severity. Image from Alzheimer's Association, 2016.
Drug Treatment
Mild to moderate stages
A main drug treatment for Alzheimer’s that targets memory loss are cholinesterase inhibitors (donepezil, rivastigmine and galantamine). For mild to moderate stages, individuals are prescribed drugs that are cholinesterase inhibitors. These inhibitors treat symptoms that relate to memory, thinking, language, judgment and all other thought processes (Alzheimer's Association, 2016). Acetylcholine is a chemical messenger important for learning and memory. It is cholinesterase inhibitors that prevent the breakdown of acetylcholine and keep the levels high (Alzheimer's Association, 2016).
Moderate to severe stages
Individuals that experiences moderate to severe stages are normally prescribed a second type of drug called memantine. Memantine is aimed to improve memory, attention, reason, language and the ability to perform simple tasks (Alzheimer's Association, 2016). It regulates the activity of glutamate, which is a different chemical messenger responsible for learning and memory (Alzheimer's Association, 2016). Another drug used to treat moderate to severe stages is Namzaric. Namzaric is a combination of memantine and donepezil. Some studies have shown that those who take a cholinesterase inhibitor such as donepezil with a combination of memantine can benefit more from it, however it is only prescribed to individuals experiencing moderate to severe stages of Alzheimer’s
Alternative Treatments
Environment
A safe and supportive environment is critical to any treatment plan. As memory demanding tasks may be difficult for individuals with Alzheimer's it is important to establish and strengthen routine habits and minimizing memory demanding tasks to make daily living more convenient to the individual (Mayo Clinic, 2015). Some examples that support individuals with Alzheimer's needs are to always place keys; wallets and phones in the same place at home so the error of misplacing valuable items does not occur. Also it can help reduce the number of mirrors in the home as the images in mirrors can confuse or frighten those with Alzheimer's. Making sure appointments that are regular are on the same day at the same time if possible to make life much easier.
Exercise and Nutrition
Exercise is used as a treatment for Alzheimer’s as it is associated with a lower risk of cognitive decline (Riordan, 2016). Physical activity increase the blood flow to the brain and body, thus providing additional nourishment that can reduce risk factors associated with Alzheimer patients such as high blood pressure, diabetes and high cholesterol (Riordan, 2016). Small activities such as walking can improve both mental and physical health as it may improve the quality of life for individuals in all stages of the condition. It is important to note that depression is very common risk associated with individuals experiencing Alzheimer’s disease and exercise is typically recommended as a treatment for such secondary symptoms. There are certain nutritional supplements that are available to specifically treat Alzheimer's disease to maintain a balanced diet. Also avoiding caffeine can prevent interference with sleep and increased restlessness.
Alternative medicine
There are many supplements that promote support for cognitive health or delay Alzheimer's but currently there is no strong evidence that this form of therapy slows down progression of cognitive decline. The following treatments, herbs and vitamins support this idea: Omega-3 fatty acids, curcumin, Ginkgo, Vitamin (Mayo Clinic, 2015). However it should be noted that dietary supplements could have serious interactions with current prescribed medication therefore it may not be practical for every patient.
Future Treatments & Prevention
Researchers are currently looking for new treatments for Alzheimer's disease specifically pertaining to studies that test new drugs to show that they can slow the progression of the disease from getting worse or at least improve the symptoms such as memory (Smith et al, 2016). Researchers are also looking for a way to diagnose Alzheimer's earlier prior to the symptoms appearing therefore these results can help with starting treatment sooner. Preventive measures are living a healthy lifestyle. However, there are many ongoing studies at the moment, such as a clinical study conducted by the Dominantly Inherited Alzheimer Network (DIAN). This study is testing if antibodies to beta-amyloid can reduce the accumulation of beta amyloid plaque in the brains of people who have the genetic mutation (Smith et al, 2016). This study allows researchers to create antibodies that reduce delays and prevent symptoms (Smith et al, 2016).
Current Clinical Trials on Potential Drug Treatments
Solanezumab
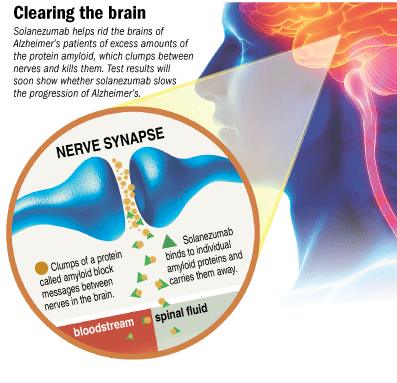
Figure 13: Pharmacodynamics of Solezumab.
Solanezumab is a humanized monoclonal IgG1 antibody that selectively binds to the soluble form of Beta-amyloid (which aggregate and form plaques in the brain) (Farlow et al, 2012). Once this binding occurs, there is a resulting efflux of Beta-amyloid protein peptides away from the Central Nervous System to the blood plasma, ultimately leading to a slowing-down of the progression of the disease.
In a randomized, double-blind, placebo-controlled clinical trial, 52 patients with AD received either a placebo treatment or the antibody/Solanezumab treatment (dosage: 100 mg every 4 weeks, 100 mg weekly, 400 mg every 4 weeks or 400 mg weekly) for 12 weeks. Safety and biomarker measurements continued until 1 year after randomization, and Magnetic Resonance Imaging and cerebrospinal fluid examinations were carried out at baseline as well as after the treatment period. The Beta-amyloid protein concentrations were measured in blood plasma as well as in the cerebrospinal fluid, and the Alzheimer’s Disease Assessment Scale – cognition (ADAS-cog; a test of cognitive performance on 11 tasks) was then delivered. Results show that the total (both bound to antibody and unbound forms) amount of Beta-amyloid in blood plasma is positively correlated with antibody/Solanezumab dosage. The total amount of Beta-amyloid in the cerebrospinal fluid also increased with the antibody treatment; however, the treatment decreased the unbound form of the protein and increased the bound form of the protein in a dose-dependent manner. These results show consistency with the pharmacodynamics of Solanezumab: clearing these amyloid proteins away from the central nervous system and essentially dumping them into the systemic circulation for later excretion. In terms of clinical effects on cognition as measured by ADAS-cog, there was no significant differences between the two treatment groups. This was expected because there would be no measurable change in cognitive performance after only 12 weeks into the treatment (note that during this time, there was no decline in the placebo group as well, showing that in the normal progression of the disease, cognitive impairment is not evident at this point just yet) (Farlow et al, 2012).
Recently, the results of a phase 3 trial of this new drug were presented at the Alzheimer’s Association International Conference 2015. The trial consisted only of individuals diagnosed with the mild form of AD. Results show that solanezumab was efficient in slowing down the progressive decline in memory and cognition with time in these patients, therefore slowing down the progression of the disease.
Carbonic Anhydrase Activators (CAAs)
Carbonic Anhydrases are a family of enzymes that catalyze the interconversion of CO2 and H2O to bicarbonate and protons; this biochemical process is critical in the maintenance of an organism’s physiological acid-base balance, as well as in the removal of carbon dioxide from tissues (Sun & Alkon, 2001). The inhibition/activation of Carbonic Anhydrases have been widely investigated in terms of their various therapeutic applications. For instance, CAAs are a new revolutionary approach towards the treatment of Alzheimer’s disease, as well as other neurodegenerative disorders that require memory therapy. Some examples of CAAs are Phenylalanine and imidazole. Carbonic Anhydrase 1 (CA1) were recorded from pyramidal cells in sections of a rat hippocampus. Application of CAAs, coupled with stimulation of cholinergic inputs from the stratum oriens and GABA-ergic inputs from stratum pyramidale at low intensities, switched the hyperpolarizing GABA-mediated inhibitory postsynaptic potentials into depolarizing postsynaptic responses, presumably due to the generation of protons as the CAAs shift the bicarbonate buffer system equation to the right. On the other hand, in the absence of CAAs, the same stimulation were not adequate to trigger the synaptic switch into depolarizing potentials. Administration of these CAAs into the intralateral ventricles triggered the rats to demonstrate an enhanced learning of the Morris water maze task; this shows that the synaptic switch is important for gating the synaptic plasticity underlying spatial memory. These findings imply that increased Carbonic Anhydrase activity through CAAs might also enhance other cognitive processes such as perception and the processing and storing of temporally associated signals. This might lead to the creation of a drug design/diagnostic tools (i.e. memory therapy) for the enhancement of synaptic efficacy, spatial learning and memory, thus potentially alleviating the memory-loss symptoms associated with Alzheimer’s disease (Sun & Alkon, 2001).
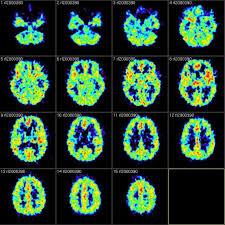
The A4 Study (Anti-Amyloid Treatment in Asymptomatic Alzheimer’s)
Figure 14: PET scans of the diseased patients.
The study is targeted towards individuals between 65-85 years old who are determined to be at risk for AD-related memory loss, but who have not yet significantly shown signs of the disease (Houston, 2015). Thus, this treatment is more of a preventative measure for those at risk. Positron Emission Tomography (PET) scans demonstrate that the Beta-amyloid plaque formation begins about 10-20 years before the initial AD symptoms start to manifest. Therefore, detecting this initial Beta-amyloid overexpression with PET scans in individuals predisposed to Alzheimer’s can then proceed to Solanezumab treatments in order to lower amyloid levels. In addition, it has been hypothesized that the accumulation of the amyloid protein may have a role in the eventual memory loss induced by AD, through an excess production of an abnormal form of the brain protein tau. This abnormal tau forms neurofibrillatory tangles that destroy nervous tissue, therefore propagating brain damage. There appears to be a point in the progression of AD where the removal of Beta-amyloid is not anymore sufficient in reversing or halting the disease development, because a critically high amount of tau has already been generated by this point. Hence, it is also crucial to use PET scans to measure the amount of abnormal tau present in the brain (Houston, 2015).
References
Abbott, A. (2011). Dementia: A problem for our age. Nature, 475(7355), S2-S4. http://dx.doi.org/10.1038/475s2a
Alzheimer's Association. (2016). Latest Medication for Memory Loss. Retrieved September 21, 2016, from http://www.alz.org/alzheimers_disease_standard_prescriptions.asp)
Chapman, D. P., Marshall Williams, S., Strine, T. W., Anda, R. F., & Moore, M. J. (2006). Dementia and Its Implications for Public Health. Preventing Chronic Disease, 3(2), A34. Retrieved 20 September 2016, from http://www.ncbi.nlm.nih.gov/pmc/art…
Del Cerro, M. & C. Triarhou, L. (2006). Remembering Alzheimer: The Man, The Disease, and the Microscope - One Hundred Years Laters. Microscopy-uk.org.uk. Retrieved 22 September 2016, from http://www.microscopy-uk.org.uk/mag…
Farlow, M., Arnold, S. E., Van Dyck, C. H., Aisen, P. S., Snider, B. J., Porsteinsson, A. P., & DeMattos, R. B. (2012). Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimer's & Dementia, 8(4), 261-271.
Hippius, H., & Neundörfer, G. (2003). The discovery of Alzheimer’s disease.Dialogues in Clinical Neuroscience, 5(1), 101–108. Retrieved 18 September 2016, from http://www.ncbi.nlm.nih.gov/pmc/art…
Houston Methodist. (2015, October 8). Preventing memory loss before symptoms appear. ScienceDaily. Retrieved September 21, 2016 from www.sciencedaily.com/releases/2015/…
Kandel, E., Schwartz, J., Jessell, T., Siegelbaum, S., & Hudspeth, A. (2013). Principles of neural science (5th ed., pp. 1328-1344). McGraw Hill Companies. (ISBN: 978-0071390118 ) Latest Medication for Memory Loss | Alzheimer's Association. (2016). Retrieved September 21, 2016, from http://www.alz.org/alzheimers_disease_standard_prescriptions.asp Leading causes of death, by sex (Both sexes). (2015). Statcan.gc.ca. Retrieved 21 September 2016, from http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/hlth36a-eng.htm
Lieff, J. (2015). The Role of Tau in Brain Function and Dementia. Searching for the Mind. Retrieved 27 December 2015, from http://jonlieffmd.com/blog/human-br…
Mahley, R., Weisgraber, K., & Huang, Y. (2006). Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proceedings Of The National Academy Of Sciences, 103(15), 5644-5651. http://dx.doi.org/10.1073/pnas.0600549103
Mayo Clinic Staff. (2015). Alzheimer's disease. Retrieved September 25, 2016, from http://www.mayoclinic.org/diseases-…
Neergaard, L. (2016). Testing brain pacemakers to zap Alzheimer's damage (Update). Medicalxpress.com. Retrieved 21 September 2016, from http://medicalxpress.com/news/2013-01-brain-pacemakers-zap-alzheimer.html
Patterson, C., Feightner, J., Garcia, A., Hsiung, G., MacKnight, C., & Sadovnick, A. (2008). Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Canadian Medical Association Journal, 178(5), 548-556. http://dx.doi.org/10.1503/cmaj.0707
Perrin, R., Fagan, A., & Holtzman, D. (2009). Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature, 461(7266), 916-922. http://dx.doi.org/10.1038/nature085…
Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer Disease and Related Neurodegenerative Diseases in Elderly Patients With Schizophrenia: A Postmortem Neuropathologic Study of 100 Cases. Arch Gen Psychiatry. 1998;55(3):205-211. doi:10.1001/archpsyc.55.3.205. Retrieved 21 September 2016, from http://archpsyc.jamanetwork.com/art…
Riordan, E. (2016). Exercise and physical activity. Retrieved September 25, 2016, from https://www.alzheimers.org.uk/site/scripts/documents_info.php?documentID=1764
Seeman, P. & Seeman, N. (2011). Alzheimer's disease: β-amyloid plaque formation in human brain. Synapse, 65(12), 1289-1297. http://dx.doi.org/10.1002/syn.20957
Scully, T. (2012). Demography: To the limit. Nature, 492(7427), S2-S3. http://dx.doi.org/10.1038/492s2a
Smith, M., Robinson, L. & Segal, J. (2016). By leading a brain-healthy lifestyle, you may be able to prevent the symptoms of Alzheimer’s disease and slow down, or even reverse, the process of deterioration. Retrieved September 25, 2016, from http://www.helpguide.org/articles/a…
Sun, M. K., & Alkon, D. L. (2001). Pharmacological enhancement of synaptic efficacy, spatial learning, and memory through carbonic anhydrase activation in rats. Journal of Pharmacology and Experimental Therapeutics, 297(3), 961-967 Understanding Genetics and Alzheimer's Disease. (2016). Retrieved 21 September 2016, from http://www.alzheimer.ca/~/media/Files/national/Research/understanding_genetics_e.pdf